Abstract
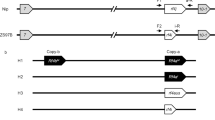
Three-line hybrid rice has primarily been developed on wild abortive (WA)-type cytoplasmic male sterility (CMS) and has helped increase the yield of rice globally. The development of WA-type CMS lines and hybrids was expedited through the identification and mapping of the fertility restorer gene (Rf) in maintainers. This study observed fertile plants in WA-TianfengA/Zhenshan97B//TianfengB population, indicating that the maintainer line ‘Zhenshan97B’ should carry Rfs for WA-type CMS. Several advanced backcross populations were generated with the genetic background of the ‘WA-TianfengA,’ and the pollen fertility levels of the backcrossed individuals in BC3F1, BC4F1 and BC4F2 populations are governed by a new gene, Rf20(t), from ‘Zhenshan97B.’ Employing bulk segregant analysis of fertile and sterile pools from the BC4F1 population, Rf20(t) was genetically mapped to a candidate region on chromosome 10. Subsequently, Rf20(t) was located between RM24883 and RM24919 through recombination analysis of molecular markers using the BC4F2 population. Implementing a substitution mapping strategy, Rf20(t) was ultimately mapped to a 245-kb region between the molecular markers STS10-122 and STS10-126 and obtained the most likely candidate gene LOC_Os10g02650, which is predicted to encode pentatricopeptide repeat-containing (PPR) protein. These results enhance our understanding of the fertility restoration of WA-type CMS lines, facilitating the development of high-quality pairs of WA-type CMS and maintainer lines.





Similar content being viewed by others
Data availability
All data generated or used during the study appear in the submitted article.
References
Abe A, Kosugi S, Yoshida K, Natsume S, Takagi H, Kanzaki H, Matsumura H, Yoshida K, Mitsuoka C, Tamiru M (2012) Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol 30:174–178
Anandakumar CR, Subramaniam S (1992) Genetics of fertility restoration in hybrid rice. Theor Appl Genet 83:994–996
Ara M, Tsuji M, Tada H (2007) Preparation of amino-terminated monolayers via hydrolysis of phthalimide anchored to Si (111). Surf Sci 601:5098–5102
Bazrkar L, Ali AJ, Babaeian NA, Ebadi AA, Allahgholipour M, Kazemitabar K, Nematzadeh G (2008) Tagging of four fertility restorer loci for wild abortive-cytoplasmic male sterility system in rice (Oryza sativa L.) using microsatellite markers. Euphytica 164:669–677
Bharaj TS, Bains SS, Sidhu GS, Gagneja MR (1991) Genetics of fertility restoration of ‘Wild Abortive’ cytoplasmic male sterility in rice, Oryza sativa L. Euphytica 56:199–203
Cai J, Liao QP, Dai ZJ, Zhu HT, Zeng RZ, Zhang ZM, Zhang GQ (2013) Allelic differentiations and effects of the Rf3 and Rf4 genes on fertility restoration in rice with wild abortive cytoplasmic male sterility. Biol Plantarum 57:274–280
Cai S, Zhang H, Zhu H, Xu S, Zhang D, Lv W (2019) Rice rotation system affects the spatial dynamics of the diazotrophic community in paddy soil of the Yangtze delta, China. Eurasian Soil Sci 52:696–706
Chen JG, Zou WL, Meng LJ, Fan XR, Xu GH, Ye GY (2019) Advances in the uptake and transport mechanisms and QTLs mapping of cadmium in rice. Int J Mol Sci 20:3417
Fujii S, Toriyama K (2009) Suppressed expression of Retrograde-Regulated Male Sterility restores pollen fertility in cytoplasmic male sterile rice plants. Proc Natl Acad Sci USA 106:9513–9518
Fu Hao-Wei, Li Y-F, Ma X-H, Shu Q-Y (2010) Study on phenotype of restoring gene with minor effect in three-line sterile lines in rice (Oryza sativa L.). Crop Res 24:8–11
Govinda RK, Virmani SS (1988) Genetics of fertility restoration of ‘WA’ type cytoplasmic male sterility in rice. Crop Sci 28:787–792
Hanson MR, Bentolila S (2004) Interactions of mitochondrial and nuclear genes that affect male gametophytic development. Plant Cell 16:154–169
Hill JT, Demarest BL, Bisgrove BW, Gorsi B, Su YC, Yost HJ (2013) MMAPPR: mutation mapping analysis pipeline for pooled RNA-seq. Genome Res 23:687–697
Hu J, Wang K, Huang W, Liu G, Gao Y, Wang J, Huang Q, Ji Y, Qin X, Wan L, Zhu R, Li S, Yang D, Zhu Y (2012) The rice pentatricopeptide repeat protein RF5 restores fertility in Hong-Lian cytoplasmic male-sterile lines via a complex with the glycine-rich protein GRP162. Plant Cell 24(1):109–122
Huang JZ, Zhi-Guo E, Zhang HL, Shu QY (2014) Workable male sterility systems for hybrid rice: Genetics, biochemistry, molecular biology, and utilization. Rice 7:1–14
Huang WC, Yu CC, Hu J, Wang LL, Dan ZW, Zhou W, He CL, Zeng YF, Yao GX, Qi JZ, Zhang ZH, Zhu RS, Chen XF, Zhu YG (2015) Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Proc Natl Acad Sci USA 112:14984–14989
Itabashi E, Iwata N, Fujii S, Kazama T, Toriyama K (2011) The fertility restorer gene, Rf2, for Lead Rice-type cytoplasmic male sterility of rice encodes a mitochondrial glycine-rich protein. Plant J 65(3):359–367
Jiang HC, Lu Q, Qiu SQ, Yu HH, Wang ZJ, Yu ZC, Lu YR, Wang L, Xia F, Wu YY, Li F, Zhang QL, Liu G, Song DD, Ma CL, Ding Q, Zhang XB, Zhang L, Zhang XT, Li X, Zhang JW, Xiao JH, Li XH, Wang NY, Ouyang YD, Zhou FS, Zhang QF (2022) Fujian cytoplasmic male sterility and the fertility restorer gene OsRf19 provide a promising breeding system for hybrid rice. P Natl Acad Sci USA 119:e2208759119
Jing RC, Li XM, Yi P, Zhu YG (2001) Mapping fertility-restoring genes of rice WA cystoplasm male sterility using SSLP markers. Bot Bull Acad Sin 42:167–171
Kazama T, Toriyama K (2014) A fertility restorer gene, Rf4, widely used for hybrid rice breeding encodes a pentatricopeptide repeat protein. Rice 7:28
Kim YJ, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Lei JC (1983) Recessive fertility restorer genes for wild male sterile in rice and the significance of its use in breeding. Fujian Agric Sci Technol 5:16–19
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Luo DP, Xu H, Liu ZL, Guo JX, Li HY, Chen LT, Fang C, Zhang QY, Bai M, Yao N, Wu H, Ji CH, Zheng HQ, Chen YL, Ye S, Li XY, Zhao XC, Li RQ, Liu YG (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet 45:573–577
Ma GH, Yuan LP (2015) Hybrid rice achievements, development and prospect in China. J Integr Agr 14:197–205
Mehrajuddin Salgotra RK, Gupta BB (2013) Interaction of restorer genes in ‘WA’-type cytoplasmic male sterility system in rice (Oryza sativa L.). Natl Acad Sci Lett 36:259–264
Ngangkham U, De S, Singh S, Parida S, Raj KK, Singh A, Mohapatra T (2010) Genic markers for wild abortive (WA) cytoplasm based male sterility and its fertility restoration in rice. Mol Breed 26:275–292
Okazaki M, Kazama T, Murata H, Motomura K, Toriyama K (2013) Whole mitochondrial genome sequencing and transcriptional analysis to uncover an RT102-type cytoplasmic male sterility-associated candidate Gene Derived from Oryza rufipogon. Plant Cell Physiol 54:1560–1568
Qaswar M, Huang J, Ahmed W, Li DC, Liu SJ, Ali S, Liu KL, Xu YM, Zhang L, Liu LS, Gao JS, Zhang HM (2019) Long-term green manure rotations improve soil biochemical properties, yield sustainability and nutrient balances in acidic paddy soil under a rice-based cropping system. Agron Basel 9:780
Rogers SO, Bendich AJ (1985) Extraction of DNA from milligram amounts of fresh, herbarium and mummified plant tissues. Plant Mol Biol 5:69–76
Shalini P, Manonmani S, Robin S (2015) Genetic analysis of fertility restoration under CGMS system in rice (Oryza sativa L.) using three-way test-cross method. J Genet 94:9–16
Sheeba N, Viraktamath B, Sivaramakrishnan S, Gangashetti M, Khera P, Sundaram R (2009) Validation of molecular markers linked to fertility restorer gene(s) for WA-CMS lines of rice. Euphytica 167:217–227
Shen Y, Cai Q, Gao M, Wang X (1996) Isolation and genetic characterization of a fertility-restoring revertant induced from cytoplasmic male sterile rice. Euphytica 90:17–23
Sun X, Liu D, Zhang X, Li W, Liu H, Hong W, Jiang C, Guan N, Ma C, Zeng H, Xu C, Song J, Huang L, Wang C, Shi J, Wang R, Zheng X, Lu C, Wang X, Zheng H (2013) SLAF-seq: an efficient method of large-scale de novo SNP discovery and genotyping using high-throughput sequencing. PLoS One 8(3):e58700
Sun HY, Qian Q, Wu K, Luo JJ, Wang SS, Zhang CW, Ma YF, Liu Q, Huang XZ, Yuan QB, Han RX, Zhao M, Dong GJ, Guo LB, Zhu XD, Gou ZH, Wang W, Wu YJ, Lin HX, Fu XD (2014) Heterotrimeric G proteins regulate nitrogen-use efficiency in rice. Nat Genet 46:652–656
Suresh PB, Srikanth B, Kishore VH, Rao IS, Vemireddy LR, Dharika N, Sundaram RM, Ramesha MS, Rao KRSS, Viraktamath B, C, Neeraja C, N, (2012) Fine mapping of Rf3 and Rf4 fertility restorer loci of WA-CMS of rice (Oryza sativa L.) and validation of the developed marker system for identification of restorer lines. Euphytica 187:421–435
Tan XL, Vanavichit A, Amornsilpa S, Trangoonrung S (1998) Genetic analysis of rice CMS-WA fertility restoration based on QTL mapping. Theor Appl Genet 97:994–999
Tang HW, Luo DP, Zhou DG, Zhang QY, Tian DS, Zheng XM, Chen LT, Liu YG (2014) The rice restorer Rf4 for wild-abortive cytoplasmic male sterility encodes a mitochondrial-localized PPR protein that functions in reduction of WA352 transcripts. Mol Plant 7:1497–1500
Waghmode BD, Mehta HD (2011) Genetics of fertility restoration of diverse cytosterile sources in rice (Oryza sativa L.). Indian J Genet 71:1–8
Wang ZH, Zou YJ, Li XY, Zhang QY, Chen LT, Wu H, Su DH, Chen YL, Guo JX, Luo D, Long YM, Zhong Y, Liu YG (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell 18:676–687
Xu ZP, Du YY, Li XX, Wang RX, Chen ZA, Zhao XQ, Liu QQ, Tang SZ, Zhang HG (2023) Identification and fine mapping of a fertility restorer gene for wild abortive cytoplasmic male sterility in the elite indica rice non-restorer line 9311. Crop J 11:887–894
Yao FY, Xu CG, Yu SB, Li JX, Gao YJ, Li XH, Zhang QF (1997) Mapping and genetic analysis of two fertility restorer loci in the wild-abortive cytoplasmic male sterility system of rice (Oryza sativa L.). Euphytica 98:183–187
You NS, Huang LX, Lei SP, Pan YQ, Lei JC (2003) Rice mini-efficient restoring gene and practice for breeding male sterile line. Acta Agric Univ Jiangxiensis 25:487–492
Zhang H, Li X, Xu Z, Wan Z, Wang R, Zhao X, Tu G, Liang G, Gu M, Tang S (2022) Precise genetic mapping of Rf18(t), a new fertility restorer gene from “Nipponbare” for wild abortive cytoplasmic male sterility in rice (Oryza sativa L.). Theor Appl Genet 135:2687–2698
Zhu YG (1979) Studies on male sterile lines of rice with different cytoplasms. Acta Agron Sin 5:29–38
Zhu L, Lu C, Shen L, Xu Y, He P, Chen Y (2008) Using doubled haploid populations of rice for quantitative trait locus mapping. In: Rice Genetics Collection-Rice Genetics III (In 2 Parts), pp 631-636
Zhuang JY, Fan YY, Wu JL, Rao ZM, Xia YW, Zheng KL (2001) Mapping genes of rice CMS-WA fertility restoration. Acta Genet Sin 28:129–134
Acknowledgments
This work was financially supported by the National Natural Science Foundation [Grant Number 32072031]; the Zhongshan Biological Breeding Laboratory [BM2022008-03]; the Promoting Project for Open Competition Mechanism to Select the Best Candidates of Jiangsu Seed Industry [grant numbers JBGS[2021] 001, JBGS[2021] 040]; the Key University Science Research Project of Jiangsu Province [Grant Number 22KJA210004]; the Modern Agriculture Foundation of Yangzhou [Grant Number SNY2022000047]; and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Funding
This study was funded by The National Natural Science Foundation [grant number 32072031]; The Zhongshan Biological Breeding Laboratory Foundation [BM2022008-03]; The Promoting Project for Open Competition Mechanism to Select the Best Candidates of Jiangsu Seed Industry [grant numbers JBGS[2021] 001, JBGS[2021] 040]; The Key University Science Research Project of Jiangsu Province [grant number 22KJA210004]; The Modern Agriculture Foundation of Yangzhou [grant number SNY2022000047]; and The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
HZ and ZX conducted the data analysis and drafted the manuscript. YL and ZX performed the phenotypic evaluation and data analysis. YD performed qRT-PCR and genome sequence. BX, ML LZ and XZ participated in the construction of the testcross populations. QL and ST participated in design of the study. HZ designed the study and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Mark E. Sorrells.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Xu, Z., Du, Y. et al. Mapping of Rf20(t), a minor fertility restorer gene for rice wild abortive cytoplasmic male sterility in the maintainer line ‘Zhenshan97B’. Theor Appl Genet 136, 248 (2023). https://doi.org/10.1007/s00122-023-04486-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04486-9