Abstract
Key message
The qHTB1-1 QTL, controlling heat tolerance at the booting stage in rice, was fine mapped to a 47.1 kb region containing eight candidate genes. Two positional candidate genes showed significant changes in expression levels under heat stress.
Abstract
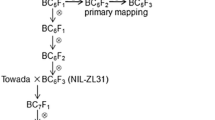
High-temperature stress at the booting stage has the potential to significantly limit rice production. An interspecific advanced backcrossed population between the Oryza sativa L. cultivar R53 and the wild Oryza rufipogon Griff accession HHT4 was used as the source material to develop a set of chromosome segment introgression lines to elucidate the genetic mechanism of the qHTB1-1 QTL in heat tolerance. A single-chromosome-segment introgression line, IL01-15, was used to develop secondary populations for the mapping of qHTB1-1 on chromosome 1 for heat tolerance at the booting stage. Using the BC5F2, BC5F3, and BC5F4 populations, we first confirmed qHTB1-1 and validated the phenotypic effect. The qHTB1-1 QTL explained 13.1%, 16.9%, and 17.8% of the phenotypic variance observed in the BC5F2, BC5F3, and BC5F4 generations, respectively. Using homozygous recombinants screened from larger BC6F2 and BC6F3 populations, qHTB1-1 was fine mapped within a 47.1 kb region between markers RM11633 and RM11642. Eight putative predicted genes were annotated in the region, and six genes were predicted to encode expressed proteins. The expression patterns of these six genes demonstrated that LOC_Os01g53160 and LOC_Os01g53220 were highly induced by heat stress in IL01-15 compared to R53. Sequence comparison of the gene-coding regions of LOC_Os01g53160 and LOC_Os01g53220 between R53 and IL01-15 revealed one synonymous and two nonsynonymous SNPs in exons, respectively. Our results provide a basis for identifying the genes underlying qHTB1-1 and indicate that markers linked to the qHTB1-1 locus can be used to improve the heat tolerance of rice at the booting stage by marker-assisted selection.








Similar content being viewed by others
References
Ashikari M, Matsuoka M (2006) Identification, isolation and pyramiding of quantitative trait loci for rice breeding. Trends Plant Sci 11:344–350
Cao L, Zhao J, Zhan X, Li D, He L, Cheng S (2003) Mapping QTLs for heat tolerance and correlation between heat tolerance and photosynthetic rate in rice. Chin J Rice Sci 17:223–227 (In Chinese with English abstract)
Chen Q, Yu S, Li C, Mou T (2008) Identification of QTLs for heat tolerance at flowering stage in rice. Sci Agric Sin 41:315–321 (In Chinese with English abstract)
Cheng L, Wang J, Uzokwe V, Meng L, Wang Y, Sun Y, Zhu L, Xu J, Li Z (2012) Genetic analysis of cold tolerance at seedling stage and heat tolerance at anthesis in rice (Oryza sativa L.). J Integr Agric 11:359–367
Confalonieri R, Rosenumund AS, Baruth B (2009) An improved model to simulate rice yield. Agron Sustain Dev 29:463–474
Jagadish SVK, Craufurd PQ, Wheeler TR (2007) High temperature stress and spikelet fertility in rice (Oryza sativa L.). J Exp Bot 58:1627–1635
Jagadish SVK, Craufurd PQ, Wheeler TR (2008) Phenotyping parents of mapping populations of rice for heat tolerance during anthesis. Crop Sci 48:1140–1146
Jagadish SVK, Muthurajan R, Oane R, Wheeler TR, Heuer S, Bennett J, Craufurd PQ (2010a) Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J Exp Bot 61:143–156
Jagadish SVK, Cairns J, Lafitte R, Wheeler TR, Price AH, Craufurd PQ (2010b) Genetic analysis of heat tolerance at anthesis in rice. Crop Sci 50:1633–1641
Jedlicka P, Mortin MA, Wu C (1997) Multiple functions of Drosophila heat shock transcription factor in vivo. EMBO J 16:2452–2462
Krishnan R, Ramakrishnan B, Reddy KR, Reddy VR (2011) High-temperature effects on rice growth, yield and grain quality. Adv Agron 111:87–206
Kui LM, Tan LB, Tu J, Lu YX, Sun CQ (2008) Identification of QTLs associated with heat tolerance of Yuanjiang common wild rice (Oryza rufipogon Griff.) at flowering stage. J Agric Biotechnol 16:461–464
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Lei D, Tan L, Liu F, Chen L, Sun C (2013) Identification of heat-sensitive QTL derived from common wild rice (Oryza rufipogon Griff.). Plant Sci 201:121–127
Li XM, Chao DY, Wu Y, Huang X, Chen K, Cui LG, Dong NQ, Guo T, Shi M, Feng Q, Zhang P, Han B, Shan JX, Gao JP, Lin HX (2015) Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat Genet 47:827
Li J, Pan Y, Guo H, Zhou L, Yang S, Zhang Z, Li Z (2018) Fine mapping of QTL qCTB10-2 that confers cold tolerance at the booting stage in rice. Theor Appl Genet 131:157–166
Ma Y, Yang C, He Y, Tian Z, Li J, Sunkar R (2017) Rice OVATE family protein 6 regulates plant development and confers resistance to drought and cold stresses. J Exp Bot 68:4885–4898
Maruyama A, Weerakoon WMW, Wakiyama Y, Ohba K (2013) Effects of increasing temperatures on spikelet fertility in different rice cultivars based on temperature gradient chamber experiments. J Agron Crop Sci 199:416–423
Matsui T, Namuco O, Ziska L, Horie T (1997a) Effect of high temperature and ‘Co2’ concentration on spikelet sterility in Indica rice. Field Crop Res 51:213–219
Matsui T, Omasa K, Horie T (1997b) High temperature induced spikelet sterility of japonica rice at flowering in relation to air humidity and wind velocity conditions. Jpn J Crop Sci 66:449–455
Matsui T, Omasa K, Horie T (2001) The differences in sterility due to high temperature during the flowering period among japonica rice varieties. Plant Prod Sci 4:90–93
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3(3):269–283
Mittal D, Chakrabarti S, Sarkar A, Singh A, Grover A (2009) Heat shock factor gene family in rice: genomic organization and transcript expression profiling in response to high temperature, low temperature and oxidative stresses. Plant Physiol Biochem 47:785–795
Nakagawa H, Horie T, Matsui T (2003) Effects of climate change on rice production and adaptive technologies. In: International rice research conference, Beijing, China, 16–19 Sept 2002. International Rice Research Institute
Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperon 6:177
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Paterson AH, De-Verna JW, Lanini B, Tanksley SD (1990) Fine mapping of quantitative trait loci using selected overlapping recombinant chromosomes, in an interspecific cross of tomato. Genetics 124:735–742
Poli Y, Basava RK, Panigrahy M, Vinukonda VP, Dokula NR, Voleti SR, Desiraju S, Neelamraju S (2013) Characterization of a Nagina22 rice mutant for heat tolerance and mapping for yield traits. Rice 6:36
Prasad P, Boote K, Allen L, Sheehy J, Thomas J (2006) Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crop Res 95:398–411
Rogers SO, Bendich AJ (1989) Extraction of DNA from plant tissues. Plant molecular biology manual. Springer, New York, pp 73–83
Satake T, Yoshida S (1978) High temperature induced sterility in Indica rice at flowering. Jpn J Crop Sci 47:6–17
Shah F, Huang J, Cui K, Nie L, Shah T, Chen C, Wang K (2011) Impact of high-temperature stress on rice plant and its traits related to tolerance. J Agric Sci 149:545–556
Shanmugavadivel PS, Sv AM, Prakash C, Ramkumar MK, Tiwari R, Mohapatra T, Singh NK (2017) High resolution mapping of QTLs for heat tolerance in rice using a 5 K SNP array. Rice 10:28
Tazib T, Kobayashi Y, Koyama H, Matsui T (2015) QTL analyses for anther length and dehiscence at flowering as traits for the tolerance of extreme temperatures in rice (Oryza sativa L.). Euphytica 203:629–642
Thanh NT, Green LA (2010) Functionalisation of nanoparticles for biomedical applications. Nano Today 5:213–230
Von Koskull-Döring P, Scharf KD, Nover L (2007) The diversity of plant heat stress transcription factors. Trends Plant Sci 12:452–457
Weerakoon WMW, Maruyama A, Ohba K (2008) Impact of humidity on temperature-induced grain sterility in rice (Oryza sativa L.). J Agron Crop Sci 194:135–140
Xiao Y, Pan Y, Luo L, Zhang G, Deng H, Dai L, Liu X, Tang W, Chen L, Wang GL (2011a) Quantitative trait loci associated with seed set under high temperature stress at the flowering stage in rice (Oryza sativa L.). Euphytica 178:331–338
Xiao YH, Pan Y, Luo LH, Deng HB, Zhang GL, Tang WB, Chen LY (2011b) Quantitative trait loci associated with pollen fertility under high temperature stress at flowering stage in rice (Oryza sativa L.). Rice Sci 18:1–7
Xu JC, Zhou LX (2002) Identification of molecular markers associated with rice root traits by correlation coefficient analysis. Yi Chuan Xue Bao 29:245–249 (In Chinese with English abstract)
Xu RR, Li R, Wang XF, Hao YJ (2018) Identification and expression analysis under abiotic stresses of OFP gene family in Apple. Sci Agric Sin 51:1948–1959 (In Chinese with English abstract)
Ye C, Argayoso MA, Redoña ED, Sierra SL, Laza MA, Dilla CJ, Mo Y, Thomson MJ, Chin J, Delaviña CB, Diaz GQ, Hernandez JE (2012) Mapping QTL for heat tolerance at flowering stage in rice using SNP markers. Plant Breed 131:33–41
Ye C, Tenorio FA, Argayoso MA, Laza MA, Koh HJ, Redoña ED, Jagadish KS, Gregorio GB (2015) Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet 16:41
Yin X, Kropff MJ, Goudriaan J (1996) Differential effects of day and night temperature on development to flowering in rice. Ann Bot 77:203–213
Yoshida S (1981) Fundamentals of rice crop science. International Rice Research Institute Los Banos, Philippines
Zhang T, Yang L, Jiang K, Huang M, Sun Q, Chen W, Zheng J (2008) QTL mapping for heat tolerance of the tassel period of rice. Mol Plant Breed 6:867–873 (In Chinese with English abstract)
Zhang G, Chen L, Xiao G, Xiao Y, Chen X, Zhang S (2009) Bulked segregant analysis to detect QTL related to heat tolerance in rice using SSR markers. Agric Sci China 8:482–487 (In Chinese)
Zhao Z, Jiang L, Xiao Y, Zhang W, Zhai H, Wan J (2006) Identification of QTLs for heat tolerance at the booting stage in rice (Oryza sativa L.). Acta Agron Sin 32:640–644 (In Chinese with English abstract)
Zhao H, Liu J, Shi L, Xu F, Wang Y (2010) Development of boron-efficient near isogenic lines of Brassica napus and their response to low boron stress at seedling stage. Russian journal of genetics 46(1):57–63
Zhao L, Lei J, Huang Y, Zhu S, Chen H, Huang R, Peng Z, Tu Q, Shen X, Yan S (2016) Mapping quantitative trait loci for heat tolerance at anthesis in rice using chromosomal segment substitution lines. Breed Sci 66:358–366
Zhu CL, Xiao YH, Wang CM, Jiang L, Zhai HQ, Wan JM (2005) Mapping QTLs for heat tolerance during grain filling in rice. Chin J Rice Sci 19:117–121
Zhu S, Huang R, Wai HP, Xiong H, Shen X, He H, Yan S (2017) Mapping quantitative trait loci for heat tolerance at the booting stage using chromosomal segment substitution lines in rice. Physiol Mol Biol Plants 23:817–825
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31560382), the Natural Science Foundation of Jiangxi Province Distinguished Young Scholars, China (Grant No. 2018ACB21026), the Natural Science Foundation of Jiangxi Province (Grant Nos. 20151BAB214013; 2017BAB204020), the Key Research and Development Program of Jiangxi Province, China (Grant No. 20161BBF60129), the Key Laboratory Project of Jiangxi Province, China (Grant No. 20181BCD40010), the Jiangxi Special Fund for Agro-scientific Research in the Collaborative Innovation, China (Grant No. JXXTCXFY201902), and the National Key R&D Program of China, China (Grant No. 2017YFD0301601; Grant No.2018YFD0301103).
Author information
Authors and Affiliations
Contributions
CZB contributed to genotyping, data analysis, and writing; LY contributed to genotyping and data collection; THW contributed to genotyping; TXY contributed to population construction; ZBH contributed to the q-PCR analysis; LQZ provided material and reviewed the manuscript; WXF contributed to population construction; CYH provided material and reviewed the manuscript; WJL and YLF contributed to the experimental design and writing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest in the reported research.
Ethical standards
The authors note that this research was performed and reported in accordance with the ethical standards for scientific conduct.
Additional information
Communicated by Albrecht E. Melchinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, Z., Li, Y., Tang, H. et al. Fine mapping of the qHTB1-1QTL, which confers heat tolerance at the booting stage, using an Oryza rufipogon Griff. introgression line. Theor Appl Genet 133, 1161–1175 (2020). https://doi.org/10.1007/s00122-020-03539-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03539-7