Abstract
Key message
We report the first study on the unique allele from wild barley that can improve waterlogging tolerance in cultivated barley with a substantially higher contribution to aerenchyma formation.
Abstract
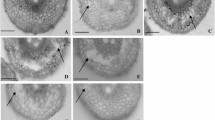
Waterlogging is one of the major abiotic stresses that dramatically reduce barley crop yield. Direct selection on waterlogging tolerance in the field is less effective due to its viability to environment. The most effective way of selection is to choose traits that make significant contributions to the overall tolerance and are easy to score. Aerenchyma formation under waterlogging stress is one of the most effective mechanisms to provide adequate oxygen supply and overcome stress-induced hypoxia imposed on plants. In this study, a new allele for aerenchyma formation was identified from a wild barley accession TAM407227 on chromosome 4H. Compared to that identified in cultivated barley, this allele not only produced a greater proportion of aerenchyma but made a greater contribution to the overall waterlogging tolerance. The QTL explained 76.8% of phenotypic variance in aerenchyma formation with a LOD value of 51.4. Markers co-segregating with the trait were identified and can be effectively used in marker assisted selection.






Similar content being viewed by others
References
Armstrong W (1979) Aeration in higher plants, vol 7. Advances in Botanical Research, London
Bertholdsson NO, Holefors A, Macaulay M, Crespo-Herrera LA (2015) QTL for chlorophyll fluorescence of barley plants grown at low oxygen concentration in hydroponics to simulate waterlogging. Euphytica 201:357–365. doi:10.1007/s10681-014-1215-0
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Broughton S, Zhou G, Teakle N, Matsuda R, Zhou M, O’Leary R, Colmer T, Li C (2015) Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare L.). Mol Breed 35:1–15. doi:10.1007/s11032-015-0243-3
Collaku A, Harrison SA (2005) Heritability of waterlogging tolerance in wheat. Crop Sci 45:722–727
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant Cell Environ 26:17–36
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36:665–681
de San Celedonio RP, Abeledo LG, Miralles D (2014) Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 378:265–277. doi:10.1007/s11104-014-2028-6
Evans DE (2004) Aerenchyma formation. New Phytol 161:35–49
Jones HG (2007) Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J Exp Bot 58:119–130. doi:10.1093/jxb/erl118
Khush GS (2001) Green revolution: the way forward. Nat Rev Genet 2:815–822
Korff M, Grando S, Del Greco A, This D, Baum M, Ceccarelli S (2008) Quantitative trait loci associated with adaptation to Mediterranean dryland conditions in barley. Theor Appl Genet 117:653–669. doi:10.1007/s00122-008-0787-2
Li H, Vaillancourt R, Mendham N, Zhou M (2008) Comparative mapping of quantitative trait loci associated with waterlogging tolerance in barley (Hordeum vulgare L.). BMC Genom 9:401. doi:10.1186/1471-2164-9-401
Malik AI, English JP, Colmer TD (2009) Tolerance of Hordeum marinum accessions to O2 deficiency, salinity and these stresses combined. Ann Bot 103:237–248. doi:10.1093/aob/mcn142
Malik AI, Islam AKMR, Colmer TD (2011) Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum–wheat amphiploids. New Phytol 190:499–508. doi:10.1111/j.1469-8137.2010.03519.x
Mano Y, Omori F (2013) Flooding tolerance in interspecific introgression lines containing chromosome segments from teosinte (Zea nicaraguensis) in maize (Zea mays subsp. mays). Ann Bot 112:1125–1139
Mano Y, Omori F (2015) Flooding tolerance in maize (Zea mays subsp. mays) F1 hybrids containing a QTL introgressed from teosinte (Zea nicaraguensis). Euphytica 205:255–267. doi:10.1007/s10681-015-1449-5
Mano Y, Muraki M, Fujimori M, Takamizo T, Kindiger B (2005) Identification of QTL controlling adventitious root formation during flooding conditions in teosinte (Zea mays ssp. huehuetenangensis) seedlings. Euphytica 142:33–42. doi:10.1007/s10681-005-0449-2
Mano Y, Omori F, Takamizo T, Kindiger B, Bird RM, Loaisiga CH (2006) Variation for root aerenchyma formation in flooded and non-flooded maize and teosinte seedlings. Plant Soil 281:269–279. doi:10.1007/s11104-005-4268-y
Rajhi I, Yamauchi T, Takahashi H, Nishiuchi S, Shiono K, Watanabe R, Mliki A, Nagamura Y, Tsutsumi N, Nishizawa NK, Nakazono M (2011) Identification of genes expressed in maize root cortical cells during lysigenous aerenchyma formation using laser microdissection and microarray analyses. New Phytol 190:351–368. doi:10.1111/j.1469-8137.2010.03535.x
Ribaut JM, Hoisington D (1998) Marker-assisted selection: new tools and strategies. Trends Plant Sci 3:236–239. doi:10.1016/S1360-1385(98)01240-0
Setter TL, Waters I (2003) Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 253:1–34
Setter TL, Waters I, Sharma SK, Singh KN, Kulshreshtha N, Yaduvanshi NP, Ram PC, Singh BN, Rane J, McDonald G, Khabaz-Saberi H, Biddulph TB, Wilson R, Barclay I, McLean R, Cakir M (2009) Review of wheat improvement for waterlogging tolerance in Australia and India: the importance of anaerobiosis and element toxicities associated with different soils. Ann Bot 103:221–235. doi:10.1093/aob/mcn137
Shabala S, Shabala L, Barcelo J, Poschenrieder C (2014) Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant Cell Environ 37:2216–2233. doi:10.1111/pce.12339
Van Ooijen JW, Kyazma BV (2009) MapQTL 6. Software for the mapping of quantitative trait loci in experimental populations of diploid species. Kyazma BV, Wageningen
Voesenek LACJ, van Veen H, Sasidharan R (2014) Learning from nature: the use of non-model species to identify novel acclimations to flooding stress. AoB Plants. doi:10.1093/aobpla/plu016
Voesenek LACJ, Sasidharan R, Visser EJW, Bailey-Serres J (2016) Flooding stress signaling through perturbations in oxygen, ethylene, nitric oxide and light. New Phytol 209:39–43. doi:10.1111/nph.13775
Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93:77–78. doi:10.1093/jhered/93.1.77
Xu R, Wang J, Li C, Johnson P, Lu C, Zhou M (2012) A single locus is responsible for salinity tolerance in a Chinese landrace barley (Hordeum vulgare L.). PLoS One 7:e43079. doi:10.1371/journal.pone.0043079
Zhang Z, Wei L, Zou X, Tao Y, Liu Z, Zheng Y (2008) Submergence-responsive MicroRNAs are potentially involved in the regulation of morphological and metabolic adaptations in maize root cells. Ann Bot 102:509–519. doi:10.1093/aob/mcn129
Zhang X, Huang X, Zhou M, Shabala L, Koutoulis A, Shabala S (2015a) Plant breeding for flood tolerance: advances and limitations. In: Dhankher OP, Jaiwal PK, Singh RP (eds) Genetic manipulation in plants for mitigation of climate change. Springer, Berlin, pp 43–72
Zhang X, Shabala S, Koutoulis A, Shabala L, Johnson P, Hayes D, Nichols D, Zhou M (2015b) Waterlogging tolerance in barley is associated with faster aerenchyma formation in adventitious roots. Plant Soil 394:355–372. doi:10.1007/s11104-015-2536-z
Zhang X, Shabala S, Koutoulis A, Shabala L, Zhou M (2016a) Meta-analysis of major QTL for abiotic stress tolerance in barley and implications for barley breeding. Planta. doi:10.1007/s00425-016-2605-4
Zhang X, Zhou G, Shabala S, Koutoulis A, Shabala L, Johnson P, Li C, Zhou M (2016b) Identification of aerenchyma formation-related QTL in barley that can be effective in breeding for waterlogging tolerance. Theor Appl Genet 129:1167–1177. doi:10.1007/s00122-016-2693-3
Zhou M (2010) Improvement of plant waterlogging tolerance. In: Mancuso S, Shabala S (eds) Waterlogging signalling and tolerance in plants. Springer, Heidelberg, pp 267–285. doi:10.1007/978-3-642-10305-6_13
Zhou M (2011) Accurate phenotyping reveals better QTL for waterlogging tolerance in barley. Plant Breed 130:203–208. doi:10.1111/j.1439-0523.2010.01792.x
Zhou M, Johnson P, Zhou G, Li C, Lance R (2012) Quantitative trait loci for waterlogging tolerance in a barley cross of Franklin× YuYaoXiangTian Erleng and the relationship between waterlogging and salinity tolerance. Crop Sci 52:2082–2088. doi:10.2135/cropsci2012.01.0008
Acknowledgements
This work was supported by the Australian Research Council Linkage grant (LP120200516) and Grains Research & Development Corporation (GRDC) of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Additional information
Communicated by Gary Muehlbauer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2017_2910_MOESM1_ESM.pptx
Supplementary Figure 1: The system of screening waterlogging tolerance in the field. A: Field preparation; B: 4 weeks after waterlogging; C: Tam407227 (left) and Franklin (right) (PPTX 975 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Fan, Y., Shabala, S. et al. A new major-effect QTL for waterlogging tolerance in wild barley (H. spontaneum). Theor Appl Genet 130, 1559–1568 (2017). https://doi.org/10.1007/s00122-017-2910-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-017-2910-8