Abstract
Key message
The wheat association mapping initiative is appropriate for gene discovery without the confounding effects of phenology and plant height.
Abstract
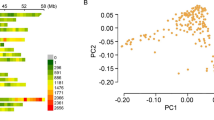
The wheat association mapping initiative (WAMI) population is a set of 287 diverse advanced wheat lines with a narrow range of variation for days to heading (DH) and plant height (PH). This study aimed to characterize the WAMI and showed that this diverse panel has a favorable genetic background in which stress adaptive traits and their alleles contributing to final yield can be identified with reduced confounding major gene effects through genome-wide association studies (GWAS). Using single nucleotide polymorphism (SNP) markers, we observed lower gene diversity on the D genome, compared with the other genomes. Population structure was primarily related to the distribution of the 1B.1R rye translocation. The narrow range of variation for DH and PH in the WAMI population still entailed segregation for a few markers associated with the former traits, while Rht genes were associated with grain yield (GY). Genotype by environment (G × E) interaction for GY was primarily explained by Rht-B1, Vrn-A1 and markers on chromosomes 2D and 3A when running GWAS with genotype scores from the G × E biplot. The use of PC scores from the G × E biplot seems a promising tool to determine genes and markers associated with complex interactions across environments. The WAMI panel lends itself to GWAS for complex trait dissection by avoiding the confounding effects of DH and PH which were reduced to a minimum (using Rht-B1 and Vrn-A1 scores as covariables), with significant associations with GY on chromosomes 2D, 3A and 3B.




Similar content being viewed by others
References
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115:721–733. doi:10.1007/s00122-007-0603-4
Bennett D, Izanloo A, Edwards J, Kuchel H, Chalmers K, Tester M, Reynolds M, Schnurbusch T, Langridge P (2012a) Identification of novel quantitative trait loci for days to ear emergence and flag leaf glaucousness in a bread wheat (Triticum aestivum L.) population adapted to southern Australian conditions. Theor Appl Genet 124:697–711. doi:10.1007/s00122-011-1740-3
Bennett D, Reynolds M, Mullan D, Izanloo A, Kuchel H, Langridge P, Schnurbusch T (2012b) Detection of two major grain yield QTL in bread wheat (Triticum aestivum L.) under heat, drought and high yield potential environments. Theor Appl Genet 125:1473–1485. doi:10.1007/s00122-012-1927-2
Berloo RV (2008) Computer note: GGT 2.0: versatile software for visualization and analysis of genetic data. J Hered 99:232–236. doi:10.1093/jhered/esm109
Bonneau J, Taylor J, Parent B, Bennett D, Reynolds M, Feuillet C, Langridge P, Mather D (2013) Multi-environment analysis and improved mapping of a yield-related QTL on chromosome 3B of wheat. Theor Appl Genet 126:747–761. doi:10.1007/s00122-012-2015-3
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet 32:314–331
Cavanagh CR, Chao S, Wang HBE, Stephen S, Kiani S, Forrest K, Saintenac C, Brown-Guedira GL, Akhunova A, See D, Bai G, Pumphrey M, Tomar L, Wong D, Kong S, Matthew Reynolds M, Lopez MS, Bockelman H, Talbert L, Anderson JA, Dreisigacker S, Baenziger S, Carter A, Korzun V, Morrell PL, Dubcovsky J, Morell MK, Sorrells ME, Hayden MJ, Akhunov E (2013) Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. Proc Nat Acad Sci 110:8062–8067
Chao S, Zhang W, Dubcovsky J, Sorrells M (2007) Evaluation of genetic diversity and genome-wide linkage disequilibrium among US wheat (Triticum aestivum L.) germplasm representing different market class. Crop Sci 47:1018–1030. doi:10.2135/cropsci2006.06.0434
Chao S, Dubcovsky J, Dvorak J, Luo MC, Baezinger SP, Matnyazov R, Clark DR, Talbert LE, Anderson JA, Dreisigacker S, Glover K, Chen J, Campbell K, Bruckner PL, Rudd JC, Haley S, Carver BF, Perry S, Sorrells ME, Akhunov ED (2010) Population and genome specific patterns of linkage disequilibrium and SNP variation in spring and winter wheat (Triticum aestivum L.). BMC Genom 11:727. doi:10.1186/1471-2164-11-727
Charmet G, Masood-Quraishi U, Ravel C, Romeuf I, Balfourier F, Perretant MR, Joseph JL, Rakszegi M, Guillon F, Sado PE, Bedo Z, Saulnier L (2009) Genetics of dietary fibre in bread wheat. Euphytica 170:155–168. doi:10.1007/s10681-009-0019-0
Chen X, Min D, Yasir TA, Hu YG (2012) Genetic diversity, population structure and linkage disequilibrium in elite Chinese winter wheat investigated with SSR markers. PLoS ONE 7:e44510. doi:10.1371/journal.pone.0044510
Crossa J, Burgueno J, Dreisigacker S, Vargas M, Herrera-Foessel SA, Lillemo M, Singh RP, Trethowan R, Warburton M, Franco J, Reynolds M, Crouch JH, Ortiz R (2007) Association analysis of historical bread wheat germplasm using additive genetic covariance of relatives and population structure. Genetics 177:1889–1913. doi:10.1534/genetics.107.078659
Diaz A, Zikhali M, Turner AS, Isaac P, Laurie DA (2012) Copy number variation affecting the photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS ONE 7(3):e33234. doi:10.1371/journal.pone.0033234
Dodig D, Zoric M, Kobiljski B, Savic J, Kandic V, Quarrie S, Barnes J (2012) Genetic association mapping study of wheat agronomic traits under contrasting water regimes. Int J Mol Sci 13:6167–6188. doi:10.3390/ijms13056167
Dreisigacker S, Shewayrga H, Crossa J, Arief VN, DeLacy IH, Singh RP, Dieters MJ, Braun HJ (2012) Genetic structures of the CIMMYT international yield trial targeted to irrigation environments. Mol Breed 29:529–541. doi:10.1007/s11032-011-9569-7
Edae EA, Byrne PF, Haley SD, Lopes MS, Reynolds MP (2014) Genome-wide association mapping of yield and yield components of spring wheat under contrasting moisture regimes. Theor Appl Genet in press doi:10.1007/s00122-013-2257-8
Ellis MH, Spielmeyer W, Gale KR, Rebetzke GJ, Richards RA (2002) Perfect markers for the Rht-B1b and Rht-D1b dwarfing genes in wheat. Theor Appl Genet 105:1038–1042. doi:10.1007/s00122-002-1048-4
Everitt B (2007) Principal component analysis. In: An R and S-Plus companion to multivariate analysis. London, Springer-Verlag pp 41–62
Fu DL, Szücs P, Yan LL, Helguera M, Skinner JS, Zitzewitz JV, Hayes PM, Dubcovsky J (2005) Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Mol Gen Genomics 273:54–65. doi:10.1007/s00438-004-1095-4
Gaut BS, Long AD (2003) The lowdown on linkage disequilibrium. Plant Cell 15:1502–1506. doi:10.1105/tpc.150730
Kang HM, Zaitlen NA, Wade CM, Kirby A, Heckerman D, Daly MJ, Eskin E (2008) Efficient control of population structure in model organism association mapping. Genetics 178:1709–1723. doi:10.1534/genetics.107.080101
Lipka AE, Tian F, Wang Q, Peiffer J, Li M, Bradbury PJ, Gore MA, Buckler ES, Zhang Z. (2012) GAPIT: genome association and prediction integrated tool. Bioinformatics pp 1–2. doi:10.1093/bioinformatics/bts444
Liu K, Muse SV (2005) PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics 21:2128–2129. doi:10.1093/bioinformatics/bti282
Liu L, He ZH, Yan J, Zhang Y, Xia X, Peña RJ (2005) Allelic variation at the Glu-1 and Glu-3 loci, presence of the 1B.1R translocation, and their effects on mixographic properties in Chinese bread wheats. Euphytica 142:197–204. doi:10.1007/s10681-005-1682-4
Lobell DB, Schlenker W, Costa-Roberts J (2011) Climate trends and global crop production since 1980. Science 333:616–620. doi:10.1126/science.1204531
Lopes MS, Reynolds MP, Jalal-Kamali MR, Moussa M, Feltaous Y, Tahir ISA, Barma N, Vargas M, Mannes Y, Baum M (2012) The yield correlations of selectable physiological traits in a population of advanced spring wheat lines grown in warm and drought environments. Field Crops Res 128:129–136. doi:10.1016/j.fcr.2011.12.017
Lopes MS, Reynolds MP, McIntyre CL, Mathews KL, Jalal Kamali MR, Mossad M, Feltaous Y, Tahir ISA, Chatrath R, Ogbonnaya F, Baum M (2013) QTL for yield and associated traits in the Seri/Babax population grown across several environments in Mexico, in the West Asia, North Africa, and South Asia regions. Theor Appl Genet 126:971–984. doi:10.1007/s00122-012-2030-4
Lopes MS, Rebetzke G, Reynolds M (2014) Integration of phenotyping and genetic platforms for a better understanding of wheat performance under drought. J Exp Bot in press doi:10.1093/jxb/eru384
Lu Y, Xu J, Yuan Z, Hao Z, Xie C, Li X, Shah T, Lan H, Zhang S, Rong T, Xu Y (2012) Comparative LD mapping using single SNPs and haplotypes identifies QTL for plant height and biomass as secondary traits of drought tolerance in maize. Mol Breeding 30:407–418. doi:10.1007/s11032-011-9631-5
Maccaferri M, Samguineti MC, Demontis A, El-Ahmed A, del Moral LG, Maalouf F, Nachit M, Nserallah N, Ouabbou H, Rhouma S, Royo C, Villegas D, Tuberosa R (2011) Association mapping in durum wheat grown across a broad range of water regimes. J Exp Bot 62:409–438. doi:10.1093/jxb/erq287
Olesen JE, Trnka M, Kersebaum KC, Skjelvåg AO, Seguin B, Peltonen-Sainio P, Rossi F, Kozyra J, Micale F (2011) Impacts and adaptation of European crop production systems to climate change. Eur J Agron 34:96–112. doi:10.1016/j.eja.2010.11.003
Patterson N, Price AL, Reich D (2006) Population structure and eigenanalysis. PLoS Genet 2:e190. doi:10.1371/journal.pgen.0020190
Pinto RS, Reynolds MP, Mathews KL, McIntyre CL, Olivares-Villegas JJ, Chapman SC (2010) Heat and drought adaptive QTL in a wheat population designed to minimize confounding. Theor Appl Genet 121:1001–1021. doi:10.1007/s00122-010-1351-4
Pritchard JK, Stephens M, Rosenberg NA, Donnelly P (2000) Association mapping in structured populations. Am J Hum Genet 67:170–181
Rebetzke GJ, Rattey AR, Farquahar GD, Richards RA, Condon AG (2012) Genomic regions of canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct Plant Biol 40:14–33
Rehman Arif MA, Nagel N, Newmann K, Kobiljski B, Lohwasser U, Borner A (2012) Genetic studies of seed longevity in hexaploid wheat using segregation and association mapping approaches. Euphytica 1869:1–13. doi:10.1007/s10681-011-0471-5
Reynolds MP, Manes Y, Izanloo A, Langridge P (2009) Phenotyping for physiological breeding and gene discovery in wheat. Ann Appl Biol 155:309–320. doi:10.1111/j.1744-7348.2009.00351.x
Richards RA, Rebetzke GJ, Watt M, Condon AG, Spielmeyer W, Dolferus R (2010) Breeding for improved water–productivity in temperate cereals: phenotyping, quantitative trait loci, markers and the selection environment. Funct Plant Biol 37:1–13
Risch N, Merikangas K (1996) The future of genetic studies of complex human diseases. Science 273:1516–1517. doi:10.1126/science.273.5281.1516
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Nat Acad Sci 81:8014–8018
Samonte SOPB, Wilson LT, McClung AM, Medley JC (2005) Targeting cultivars onto rice growing environments using AMMI and SREG GGE biplot analysis. Crop Sci 45:2414–2424. doi:10.2135/cropsci2004.0627
Schwarz EG (1978) Estimating the dimension of a model. Ann Stat 6:461–464. doi:10.1214/aos/1176344136
Sharma RC, Crossa J, Velu G, Huerta-Espino J, Vargas M, Payne TS, Singh RP (2012) Genetic gains for grain yield in CIMMYT spring bread wheat across international environments. Crop Sci 52:1522–1533. doi:10.2135/cropsci2011.12.0634
Trevaskis B, Bagnall DJ, Ellis MH, Peacock WJ, Dennis ES (2003) MADS box genes control vernalization-induced flowering in cereals. Proc Natl Acad Sci USA 100:130–13104. doi:10.1073/pnas.1635053100
Weir BS (1996) Genetic data analysis II. Sinauer Associates, Sunderland
Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.). Theor Appl Genet 118:285–294. doi:10.1007/s00122-008-0898-9
Yan W, Hunt LA (2001) Interpretation of genotype x environment interaction for winter wheat yield in Ontario. Crop Sci 41:19–25. doi:10.2135/cropsci2001.41119x
Yan LL, Helguera M, Kato K, Fukuyama S, Sherman J, Dubcovsky J (2004) Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor Appl Genet 109:1677–1686. doi:10.1007/s00122-004-1796-4
Zhang P, Dreisigacker S, Melchinger AE, Reif JC, Mujeeb Kazi A, Van Ginkel M, Hoisington D, Warburton ML (2005) Quantifying novel sequence variation and selective advantage in synthetic hexaploid wheats and their backcross-derived lines using SSR markers. Mol Breed 15:1–10. doi:10.1007/s00122-004-1796-4
Zhang LY, Liu DC, Guo XL, Yang WL, Sun JZ, Wang DW (2011) Investigation of genetic diversity and population structure of common wheat cultivars in northern China using DArT markers. BMC Genet 12:42. doi:10.1186/1471-2156-12-42
Acknowledgments
Authors would like to thank Mayra Barcelo, Araceli Torres and Eugenio Perez for assistance with data and trial management. Editing assistance was received from Emma Quilligan. The German Federal Ministry for Economy Cooperation and Development (BMZ) and the Australian Grains Research and Development Corporation (GRDC) are acknowledged for their financial support.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This experiment complies with the current laws of Mexican authorities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Peter Langridge.
Electronic supplementary material
Below is the link to the electronic supplementary material.
122_2014_2444_MOESM1_ESM.pptx
Supplementary Fig. 1- Q–Q plots (cumulative distribution of observed association p values versus expected association p values against a theoretical cumulative distribution function of no association represented by a red straight line, X = Y all log transformed) for grain yield (GY), days to heading (DH) and plant height (PH) measured under IRRIGATED, DROUGHT, HEAT and heat combined with drought (HD) conditions. QQ plots are also shown for average of all traits across all environments (AVG), average across stress environments (AVG STRESS), and PCs resulting from the PC scores of the genotype by environment interaction biplot. Blue circles indicate observed association p values and gray bar corresponds to 95 % confidence interval for the Q–Q plot under the null hypothesis of no association between the SNP and the trait. Q–Q plots are only shown for the traits showing significant associated markers. (PPTX 447 kb)
122_2014_2444_MOESM2_ESM.xlsx
Supplementary Table 1- SNP markers associated with the Rht-B1, Rht-D1, Vrn-A1, Vrn-B1, and Vrn-D1 functional genes. SNP markers shown had the highest–log q association values for the former functional genes. SNP marker, chromosome (Chr) and position (Pos) where these were located are shown for each functional gene. GWAS was conducted with scores obtained for each functional marker. (XLSX 10 kb)
122_2014_2444_MOESM3_ESM.xlsx
Supplementary Table 2- Evaluation GWAS models for grain yield (GY), days to heading (DH) and plant height (PH) measured in four environments under IRRIGATED, DROUGHT, HEAT, heat combined with drought (HD), average across stress environments (AVG STRESS with averages of DROUGHT, HEAT and HD environments), average across all environments (AVG), and scores of genotype by environment interaction biplot (PC1 and PC2 from site regression). Two sets of models were tested for each trait: 1) no correction was applied in the GWAS besides the kinship and PCs; 2) scores for Rht and Vrn functional genes included as covariates for correction of their effects in GWAS plus kinship and PCs resulting from population structure analysis; The best model is highlighted in yellow and corresponds to the highest BIC (Schwarz, 1978). (XLSX 14 kb)
122_2014_2444_MOESM4_ESM.xlsx
Supplementary Table 3- SNP markers associated with grain yield (GY) by GWAS in the WAMI grown under 4 environments (IRRIGATED, DROUGHT, HEAT and heat combined with drought, HD), calculated as an average of all environments (AVG), calculated as an average of stress environments (AVG STRESS) and PC scores obtained in the genotype by environment biplot. GWAS results are shown without and with correction by Vrn and Rht as covariables (“NO CORRECTION” and “CORRECTION”, respectively). SNP marker associated with trait, chromosome (Chr), position, -log(p value), major allele frequency (maf), R2 with and without the marker, false discovery rate (FDR) and estimates of allelic effects for each marker (Allelic Eff). *, position determined by marker–marker association. (XLSX 14 kb)
122_2014_2444_MOESM5_ESM.xlsx
Supplementary Table 4- SNP markers associated with days to heading (DH) by GWAS in the WAMI grown under 4 environments (IRRIGATED, DROUGHT, HEAT and heat combined with drought, HD), calculated as an average of all environments (AVG), calculated as an average of stress environments (AVG STRESS) and PC scores obtained in the genotype by environment biplot. GWAS results are shown without and with correction by Vrn and Rht as covariables (“NO CORRECTION” and “CORRECTION”, respectively). SNP marker associated with trait, chromosome (Chr), position, -log(p value), major allele frequency (maf), R2 with and without the marker, false discovery rate (FDR) and estimates of allelic effects for each marker (Allelic Eff). *, position determined by marker–marker association. (XLSX 14 kb)
122_2014_2444_MOESM6_ESM.xlsx
Supplementary Table 5- SNP markers associated with plant height (PH) by GWAS in the WAMI grown under 4 environments (IRRIGATED, DROUGHT, HEAT and heat combined with drought, HD), calculated as an average of all environments (AVG), calculated as an average of stress environments (AVG STRESS) and PC scores obtained in the genotype by environment biplot. GWAS results are shown without and with correction by Vrn and Rht as covariables (“NO CORRECTION” and “CORRECTION”, respectively). SNP marker associated with trait, chromosome (Chr), position, -log(p value), major allele frequency (maf), R2 with and without the marker, false discovery rate (FDR) and estimates of allelic effects for each marker (Allelic Eff). *, position determined by marker–marker association. (XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Lopes, M.S., Dreisigacker, S., Peña, R.J. et al. Genetic characterization of the wheat association mapping initiative (WAMI) panel for dissection of complex traits in spring wheat. Theor Appl Genet 128, 453–464 (2015). https://doi.org/10.1007/s00122-014-2444-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-014-2444-2