Abstract
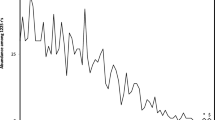
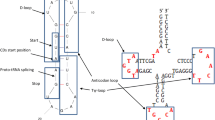
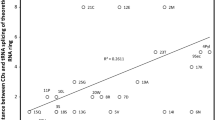
Deaminations (A->G, C->T) increase with DNA singlestrandedness during replication, presumably creating spontaneous genomic mutational and nucleotide frequency gradients. Alternatively, genes are positioned to avoid deaminations. Deamination gradients affect directly mitogene third codon positions; conserved vertebrate mitochondrial tRNA and protein coding gene arrangements minimize deaminations in anticodons, and first and second codon positions in mitogenes. Here we describe deamination gradients across theoretical minimal RNA rings, 22 nucleotide-long RNAs designed to simulate prebiotic RNAs. These RNA rings code for a start/stop codon and a single codon for each amino acid, and form stem-loop hairpins slowing degradation. They resemble consensus tRNAs, defining potential anticodons and cognate amino acids. Theoretical minimal RNA rings are not designed to include deamination gradients, yet deamination gradients occur in RNA rings. tRNA homology produces stronger evidence for deamination gradients than RNA ring homology defined by coding properties. Deamination gradients start at predicted RNA ring anticodons, corresponding to known homologies between mitochondrial tRNAs and replication origins, and between bacterial tRNA synthetases and mitochondrial DNA polymerase gamma. Deamination gradients are strongest for RNA rings with predicted anticodons matching cognate amino acids that integrated early the genetic code. Presumably protections against deaminations evolved while amino acids integrated the genetic code. Results confirm tRNA-RNA ring homologies. Coding constraints defining RNA rings presumably produce deamination gradients starting at predicted anticodons. Hence, the universal genetic code determines nucleotide deamination gradients in theoretical minimal RNA rings, suggesting adaptation to prevent consequences of deamination mutations. Results also indicate that the genetic code’s structure determined evolution of tRNAs, their cognates, tRNA synthetases, and polymerases.



Similar content being viewed by others
References
Agmon IC (2016) Could a proto-ribosome emerge spontaneously in the prebiotic world? Molecules 21:e1701
Arquès DG, Michel CJ (1996) A complementary circular code in the protein coding genes. J Theor Biol 182:45–58
Barthélémy RM, Seligmann H (2016) Cryptic tRNAs in chaetognath mitochondrial genomes. Comput Biol Chem 62:119–132
Basu S, Majumdar R, Das GK, Bhattacharyya D (2005) Energy barriers and rates of tautomeric transitions in DNA bases: ab initio quantum chemical study. Indian J Biochem Biophys 42:378–385
Bhagwat AS, Hao W, Townes JP, Lee H, Tang H, Foster PL (2016) Strand-biased cytosine deamination at the replication fork causes cytosine to thymine mutations in Escherichia coli. Proc Natl Acad Sci U S A 113:2176–2181
Bielawski JP, Gold JR (2002) Mutation patterns of mitochondrial H- and L-strand DNA in closely related Cyprinid fishes. Genetics 161:1589–1597
Blake RD, Hess ST, Nicholson-Tuell J (1992) The influence of nearest neighbors on the rate and pattern of spontaneous point mutations. J Mol Evol 34:189–200
Bloch DP, McArthur B, Widdowson R, Spector D, Guimaraes RC, Smith J (1983) tRNA-rRNA sequence homologies: evidence for a common evolutionary origin? J Mol Evol 19:420–428
Bloch D, McArthur B, Widdowson R, Spector D, Guimaraes RC, Smith J (1984) tRNA-rRNA sequence homologies: a model for the origin of a common ancestral molecule, and prospects for its reconstruction. Orig Life 14:571–578
Bloch DP, McArthur B, Mirrop S (1985) RNA-rRNA sequence homologies: evidence for an ancient modular format shared by tRNAs and rRNAs. Biosystems 17:209–225
Bloch DP, McArthur B, Guimarães RC, Smith J, Staves MP (1989) tRNA-rRNA sequence matches from inter- and intraspecies comparisons suggest common origins for the two RNAs. Braz J Med Biol Res 22:931–944
Boore JL (2000) The duplication/random loss model for gene rearrangement exemplified by mitochondrial genomes of deuterostome animals. In: Sankoff D, Nadeau J (eds) Computational biology series, 1st edn. Kluwer Academic Publishers, Dordrecht, pp 133–147
Brovarets O, Hovorun DM (2018) Renaissance of the tautomeric hypothesis of the spontaneous point mutations in DNA: new ideas and computational approaches. In Mitochondrial DNA: new insights, Seligmann H, Warthi G, editors. Chapter 2. IntechOpen, DOI: https://doi.org/10.5772/intechopen.72029
Burrows CJ, Muller JG (1998) Oxidative nucleobase modifications leading to strand scission. Chem Rev 98:1109–1152
Caetano-Anollés G (2002) Tracing the evolution of RNA structure in ribosomes. Nucleic Acids Res 30:2575–2587
Caetano-Anollés D, Caetano-Anollés G (2016) Piecemeal buildup of the genetic code, ribosomes, and genomes from primordial tRNA building blocks. Life (Basel) 6:e43
Caetano-Anollés G, Sun FJ (2014) The natural history of transfer RNA and its interactions with the ribosome. Front Genet 5:127
Carrodeguas JA, Kobayashi R, Lim SE, Copeland WC, Bogenhagen DF (1999) The accessory subunit of Xenopus laevis mitochondrial DNA polymerase gamma increases processivity of the catalytic subunit of human DNA polymerase gamma and is related to class II aminoacyl-tRNA synthetases. Mol Cell Biol 19:4039–4046
Chaley MB, Korotkov EV, Phoenix DA (1999) Relationships among isoacceptor tRNAs seem to support the co-evolution theory of the origin of the genetic code. J Mol Evol 48:168–177
Chen S, Goode AE, Skepper JN, Thorley AJ, Seiffert JM, Chung KF, Tetley TD, Shaffer MSP, Ryan MP, Porter AE (2015) Avoiding artefacts during electron microscopy of silver nanomaterials exposed to biological environments. J Microsc 261:157–166
Chu XL, Zhang BW, Zhang QG, Zhu BR, Lin K, Zhang DY (2018) Temperature responses of mutation rate and mutational spectrum in an Escherichia coli strain and the correlation with metabolic rate. BMC Evol Biol 18:126
Ciesielski GL, Bermek O, Rosado-Ruiz FA, Hovde SL, Naitzke OJ, Griffith JD, Kaguni LS (2015) Mitochondrial single-stranded DNA-binding proteins stimulate the activity of DNA polymerase γ by organization of the template DNA. J Biol Chem 290:28697–28707
Colson P, Ravaux I, Tamalet C, Glazunova O, Baptiste E, Chabriere E, Wiedemann A, Lacabaratz C, Chefrour M, Picard C, Stein A, Levy Y, Raoult D (2014) HIV infection en route to endogenization: two cases. Clin Microbiol Infect 20:1280–1288
Demongeot J (1978) Sur la possibilité de considérer le code génétique comme un code à enchaînement. Revue de Biomaths 62:61–66
Demongeot J, Besson J (1983) Genetic-code and cyclic codes. Comptes R Acad Sci III Life Sci 296:807–810
Demongeot J, Moreira A (2007) A possible circular RNA at the origin of life. J Theor Biol 249:314–324
Demongeot J, Seligmann H (2019a) Theoretical minimal RNA rings recapitulate the order of the genetic code's codon-amino acid assignments. J Theor Biol 471:108–116
Demongeot J, Seligmann H (2019b) More pieces of ancient than recent theoretical minimal proto-tRNA-like RNA rings in genes coding for tRNA synthetases. J Mol Evol. https://doi.org/10.1007/s00239-019-09892-6
Demongeot J, Seligmann H (2019c) Bias for 3′-dominant codon directional asymmetry in theoretical minimal RNA rings. J Comput Biol, in press
Demongeot J, Seligmann H (2019d) Spontaneous evolution of circular codes in theoretical minimal RNA rings. Gene 705:95–102. https://doi.org/10.1016/j.gene.2019.03.069
Demongeot J, Aracena J, Ben Lamine S, Mermet MA, Cohen O (2000) Hot spots in chromosomal breakage: from description to etiology. In: Sankoff D, Nadeau J (eds) Computational biology series, 1st edn. Kluwer Academic Publishers, Dordrecht, pp 71–85
Dufton MJ (1997) Genetic code synonym quotas and amino acid complexity: cutting the cost of proteins? J Theor Biol 187:165–173
Eigen M (1980) Das Urgen. Nova Acta Leopoldina 243. Deutsche Akademie der Naturforscher Leopoldina, Halle
Eigen M, Winkler-Oswatitsch R (1981a) Transfer-RNA: the early adaptor. Naturwissenschaften 68:217–228
Eigen M, Winkler-Oswatitsch R (1981b) Transfer-RNA, an early gene? Naturwissenschaften 68:282–292
El Houmami N, Seligmann H (2017) Evolution of nucleotide punctuation marks: from structural to linear signals. Front Genet 8:36
Elzanowski A, Ostell J (2019) The genetic codes. National Center for Biotechnology Information (NCBI), Bethesda, Maryland, USA. https://www.ncbi.nlm.nih.gov/Taxonomy/Utils/wprintgc.cgi?chapter=tgencodes#SG1. Accessed April 2019
Fan L, Sanschagrin PC, Kaguni LS, Kuhn LA (1999) The accessory subunit of mtDNA polymerase shares structural homology with aminoacyl-tRNA synthetases: implications for a dual role as a primer recognition factor and processivity clamp. Proc Natl Acad Sci U S A 96:9527–9532
Farias ST, Rêgo TG, José MV (2014) Origin and evolution of the peptidyl transferase center from proto-tRNAs. FEBS Open Bio 4:175–178
Farias ST, Rêgo TG, José MV (2019) Origin of the 16S ribosomal molecule from ancestor tRNAs. Sci 1:8
Faure E, Delaye L, Tribolo S, Levasseur A, Seligmann H, Barthélémy R-M (2011) Probable presence of an ubiquitous cryptic mitochondrial gene on the antisense strand of the cytochrome oxidase I gene. Biol Direct 6:56
Fimmel E, Michel CJ, Starman M, Strüngmann LH (2018) Self-complementary circular codes in coding theory. Theory in Biosci 137:51–65
Fonseca MM, Posada D, Harris DJ (2008) Inverted replication of vertebrate mitochondria. Mol Biol Evol 22:805–808
Fonseca MM, Harris DJ, Posada D (2014) The inversion of the control region in three mitogenomes provides further evidence for an asymmetric model of vertebrate mtDNA replication. PLoS One 9:e106654
Francino MP, Ochman H (2001) Deamination as the basis of strand-asymmetric evolution in transcribed Escherichia coli sequences. Mol Biol Evol 18:1147–1150
Francino MP, Chao L, Riley MA, Ochman H (1996) Asymmetries generated by transcription-coupled repair in enterobacterial genes. Science 272:107–109
Frederico A, Kunkel TA, Shaw BR (1990) A sensitive genetic assay for the detection of cytosine deamination: determination of rate constants and the activation energy. Biochemistry 29:2532–2537
Frederico A, Kunkel TA, Shaw BR (1993) Cytosine deamination in mismatched base-pairs. Biochemistry 32:6523–6530
Freeland SJ, Hurst LD (1998) The genetic code is one in a million. J Mol Evol 47:238–248
Grigoriev A (1999) Strand-specific compositional asymmetries in double-stranded DNA viruses. Virus Res 60:1–19
Guimarães RC (2011) Metabolic basis for the self-referential genetic code. Orig Life Evol Biosph 41:357–371
Guimarães RC (2014) Essentials in the life process indicated by the self-referential genetic code. Orig Life Evol Biosph 44:269–277
Guimarães RC (2015) The self-referential genetic code is biologic and includes the error minimization property. Orig Life Evol Biosph 45:69–75
Guimarães RC (2017) Self-referential encoding on modules of anticodon pairs—roots of the biological flow system. Life 7:16
Guimarães RC, Moreira CH, de Farias ST (2008) A self-referential model for the formation of the genetic code. Theory Biosci 127:249–270
Han DX, Wang HY, Ji ZL (2010) Amino acid homochirality may be linked to the origin of phosphate-based life. J Mol Evol 70:577–582
Hixson JE, Wong TW, Clayton DA (1986) Both the conserved stem-loop and divergent 5′-flanking sequences are required for initiation at the human mitochondrial origin of light-strand DNA replication. J Biol Chem 261:2384–2390
Hoyle F, Wickramasinghe NC (1999) Panspermia 2000. Astrophys Space Sci 268:1–17
Itzkovitz S, Alon U (2007) The genetic code is nearly optimal for allowing additional information within protein-coding sequences. Genome Res 17:405–412
Johnson DBF, Wang L (2010) Imprints of the genetic code in the ribosome. Proc Natl Acad Sci U S A 107:8298–8303
Kino K, Hirao-Suzuki M, Morikawa M, Sakaga A, Miyazawa H (2017) Generation, repair and replication of guanine oxidation products. Genes Environ 39:21
Knisbacher BA, Levanon EY (2016) DNA editing of LTR retrotransposons reveals the impact of APOBECs on vertebrate genomes. Mol Biol Evol 33:554–567
Kouno T, Silvas TV, Hilbert BJ, Shandilya SMD, Bohn MF, Kelch BA, Royer WE, Somasundaran M, Kurt Yilmaz N, Matsuo H, Schiffer CA (2017) Crystal structure of APOBEC3A bound to single-stranded DNA reveals structural basis for cytidine deamination and specificity. Nat Commun 8:15024
Krishnan NM, Rao BJ (2009) A comparative approach to elucidate chloroplast genome replication. BMC Genomics 10:237
Krishnan NM, Seligmann H, Raina SZ, Pollock DD (2004a) Detecting gradients of asymmetry in site-specific substitutions in mitochondrial genomes. DNA Cell Biol 23:707–714
Krishnan NM, Seligmann H, Raina SZ, Pollock DD (2004b) Phylogenetic analyses detect site-specific perturbations in asymmetric mutation gradients. Curr Comput Mol Biol 2004:266–267
Kumar B, Saini S (2016) Analysis of the optimality of the standard genetic code. Mol BioSyst 12:2642–2651
Kvenvolden KA, Lawless JG, Ponnamperuma C (1971) Nonprotein amino acids in the Murchison meteorite. Proc Natl Acad Sci U S A 68:486–490
Li WH, Wu CI, Luo CC (1984) Nonrandomness of point mutation as reflected in nucleotide substitutions in pseudogenes and its evolutionary implications. J Mol Evol 21:58–71
Maizels N, Weiner AM (1994) Phylogeny from function: evidence from the molecular fossil record that tRNA originated in replication, not translation. Proc Natl Acad Sci U S A 91:6729–6734
McFadden, J (2016) Quantum Leap: Could quantum mechanics hold the secret of (Alien) life? In , Ed. Al-Kahalili, J, Aliens, Profile Books Ltd, London, 2016
McPherson P, Barone F, Maga G, Mazzei M, Karran P, Bignami M (2005) 8-oxoguanine incorporation into DNA repeats in vitro and mismatch recognition by MutSα. Nucleic Acids Res 33:5094–5105
Michel CJ (2019) Single-frame, multiple-frame and framing motifs in genes. Life (Basel) 9:E18
Michel CJ, Seligmann H (2014) Bijective transformation circular codes and nucleotide exchanging RNA transcription. Biosystems 118:39–50
Michel CJ, Pellegrini M, Pirillo G (2016) Maximal dinucleotide and trinucleotide circular codes. J Theor Biol 389:40–46
Miller SL (1953) Production of amino acids under possible primitive earth conditions. Science 117:528–529
Minetti G, Egée S, Mörsdorf D, Steffen P, Makhro A, Achilli C, Ciana A, Wang J, Bouyer G, Bernhardt I, Wagner C, Thomas S, Bogdanova A, Kaestner L (2013) Red cells investigations: art and artefacts. Blood Rev 27:91–101
Morikawa M, Kino K, Oyoshi T, Suzuki M, Kobayashi T, Miyazawa H (2014) Analysis of guanine oxidation products in double-stranded DNA and proposed guanine oxidation pathways in single-stranded, double-stranded or quadruplex DNA. Biomolecules 4:140–159
Murakami E, Feng JY, Lee H, Hanes J, Johnson KA, Anderson KS (2003) Transcriptase and other RNA-associated catalytic activities by human DNA polymerase γ: importance in mitochondrial DNA replication. J Biol Chem 278:36403–36409
Mushegian AR, Koonin EV (1996) A minimal gene set for cellular life derived by comparison of complete bacterial genomes. Proc Natl Acad Sci U S A 93:10268–10273
Opuu V, Silvert M, Simonson T (2017) Computational design of fully overlapping coding schemes for protein pairs and triplets. Sci Rep 7:15873
Pelc SR (1965) Correlation between coding-triplets and amino acids. Nature 207:597–599
Pelc SR, Welton MGE (1966) Stereochemical relationship between coding triplets and amino-acids. Nature 209:868–870
Poeschla E (2013) The importance of becoming double-stranded: innate immunity and the kinetic model of HIV-1 central plus strand synthesis. Virology 441:1–11
Popov O, Segal DM, Trifonov EN (1996) Linguistic complexity of protein sequences as compared to texts of human languages. Biosystems 38:65–74
Raina SZ, Faith JJ, Disotell TR, Seligmann H, Stewart CB, Pollock DD (2005) Evolution of base-substitution gradients in primate mitochondrial genomes. Genome Res 15:665–673
Rastogi V, Puri N, Arora S, Kaur G, Yadav L, Sharma R (2013) Artefacts: a diagnostic dilemna – a review. J Clin Res 7:2408–2413
Reyes A, Gissi C, Pesole G, Saccone C (1998) Asymmetrical directional mutation pressure in the mitochondrial genome of mammals. Mol Biol Evol 15:957–966
Richardson SR, Narvaiza I, Planegger RA, Weitzman MD, Moran JV (2014) APOBEC3A deaminates transiently exposed single-strand DNA during LINE-1 retrotransposition. Elife 3:e02008
Rogers SO (2019) Evolution of the genetic code based on conservative changes of codons, amino acids, and aminoacyl tRNA synthetases. J Theor Biol 466:1–10
Schroeder JW, Hirst WG, Szezcyk GA, Simmons LA (2016) The effect of local sequence context on mutational bias of genes encoded on the leading and lagging strands. Curr Biol 26:692–697
Seligmann H (2006) Error propagation across levels of organization: from chemical stability of ribosomal RNA to developmental stability. J Theor Biol 242:69–80
Seligmann H (2008) Hybridization between mitochondrial heavy strand tDNA and expressed light strand tRNA modulates the function of heavy strand tDNA as light strand replication origin. J Mol Biol 379:188–199
Seligmann H (2010) Mitochondrial tRNAs as light strand replication origins: similarity between anticodon loops and the loop of the light strand replication origin predicts initiation of DNA replication. BioSystems 99:85–93
Seligmann H (2011) Mutation patterns due to converging mitochondrial replication and transcription increase lifespan, and cause growth rate-longevity tradeoffs. In: Seligmann H (ed) DNA Replication-Current Advances. ISBN: 978-953-307-5
Seligmann H (2012a) Replicational mutation gradients, dipole moments, nearest neighbour effects and DNA polymerase gamma fidelity in human mitochondrial genomes. In Stuart D (ed) The mechanisms of DNA replication. ISBN: 978-953-51-0991-4
Seligmann H (2012b) Coding constraints modulate chemically spontaneous mutational replication gradients in mitochondrial genomes. Curr Genomics 13:37–54
Seligmann H (2012c) Overlapping genetic codes for overlapping frameshifted genes in Testudines, and Lepidochelys olivacea as special case. Comput Biol Chem 41:18–34
Seligmann H (2015a) Phylogeny of genetic codes and punctuation codes within genetic codes. Biosystems 129:36–43
Seligmann H (2015b) Codon expansion and systematic transcriptional deletions produce tetra-, pentacoded mitochondrial peptides. J Theor Biol 387:154–165
Seligmann H (2016a) Swinger RNA self-hybridization and mitochondrial non-canonical swinger transcription, transcription systematically exchanging nucleotides. J Theor Biol 399:84–91
Seligmann H (2016b) Translation of mitochondrial swinger RNAs according to tri-, tetra- and pentacodons. Biosystems 140:38–48
Seligmann H (2016c) Natural chymotrypsin-like-cleaved human mitochondrial peptides confirm tetra-, pentacodon, non-canonical RNA translations. BioSystems 147:78–93
Seligmann H (2016d) Unbiased mitoproteome analyses confirm non-canonical RNA, expanded codon translations. Comput Struct Biotechnol J 14:391–403
Seligmann H (2016e) Chimeric mitochondrial peptides from contiguous regular and swinger RNA. Comput Struct Biotechnol J 14:283–297
Seligmann H (2017a) Natural mitochondrial proteolysis confirms transcription systematically exchanging/deleting nucleotides, peptides coded by expanded codons. J Theor Biol 414:76–90
Seligmann H (2017b) Reviewing evidence for systematic transcriptional deletions, nucleotide exchanges, and expanded codons, and peptide clusters in human mitochondria. BioSystems 160:10–24
Seligmann H (2018a) Alignment-based and alignment-free methods converge with experimental data on amino acids coded by stop codons at split between nuclear and mitochondrial genetic codes. Biosystems 167:33–46
Seligmann H (2018b) Directed mutations recode mitochondrial genes: from regular to stopless genetic codes. Chapter in: Seligmann H (ed) Mitochondrial DNA – new insights. InTechOpen, ISBN: 978-1-78984-265-4
Seligmann H (2018c) Giant viruses as protein-coated amoeban mitochondria? Virus Res 253:77–86
Seligmann H (2018d) Protein sequences recapitulate genetic code evolution. Comput Struct Biotechnol J 16:177–189
Seligmann H (2019) Giant viruses: spore-like intermediates between Rickettsia and mitochondria? Acad Sci, Ann NY in press
Seligmann H, Amzallag GN (2002) Chemical interactions between amino acid and RNA: multiplicity of the levels of specificity explains origin of the genetic code. Naturwissenschaften 89:542–551
Seligmann H, Krishnan NM (2006) Mitochondrial replication origin stability and propensity of adjacent tRNA genes to form putative replication origins increase developmental stability in lizards. J Exp Zool B Mol Dev Evol 306:433–449
Seligmann H, Labra A (2014) The relation between hairpin formation by mitochondrial WANCY tRNAs and the occurrence of the light strand replication origin in Lepidosauria. Gene 542:248–257
Seligmann H, Raoult D (2016) Unifying view of stem-loop hairpin RNA as origin of current and ancient parasitic and non-parasitic RNAs, including in giant viruses. Curr Opin Microbiol 31:1–8
Seligmann H, Raoult D (2018) Stem-loop RNA hairpins in giant viruses: invading rRNA-like repeats and a template-free RNA. Front Microbiol 9:101
Seligmann H, Warthi G (2017) Genetic code optimization for cotranslational protein folding: codon directional asymmetry correlates with antiparallel betasheets, tRNA synthetase classes. Comput Struct Biotech J 15: 414–424
Seligmann H, Krishnan NM, Rao BJ (2006a) Possible multiple origins of replication in primate mitochondria: alternative role of tRNA sequences. J Theor Biol 241:321–332
Seligmann H, Krishnan NM, Rao BJ (2006b) Mitochondrial tRNA sequences as unusual replication origins: pathogenic implications for Homo sapiens. J Theor Biol 243:375–385
Sharma S, Wang J, Alqassim E, Portwood S, Cortes Gomez E, Maguire O, Basse PH, Wang ES, Segal BH, Baysal BE (2019) Mitochondrial hypoxic stress induces widespread RNA editing by APOBEC3G in natural killer cells. Genome Biol 20:37
Sohail A, Klapacz J, Samaranayake M, Ullah A, Bhagwat AS (2003) Human activation-induced cytidine deaminase causes transcription dependent, strand-biased C to U deaminations. Nucl. Acids Res 31:2990–2994
Steele EJ (2017) Reverse transcriptase mechanism of somatic hypermutation: 60 years of clonal selection theory. Front Immunol 8:1611
Steele EJ, Lindley RA (2017) ADAR deaminase A-to-I editing of DNA and RNA moieties of RNA:DNA hybrids has implications for the mechanism of Ig somatic hypermutation. DNA Repair 55:1–6
Strauss C, Long H, Patterson CE, Te R, Lynch M (2017) Genome-wide mutation rate response to pH change in the coral reef pathogen Vibrio shilonii AK1. mBio 8:e01021–e01017
Sun FJ, Caetano-Anollés G (2008a) The origin and evolution of tRNA inferred from phylogenetic analysis of structure. J Mol Evol 66:21–35
Sun FJ, Caetano-Anollés G (2008b) Evolutionary patterns in the sequence and structure of transfer RNA: early origins of archaea and viruses. PLoS Comput Biol 4:e1000018
Sun FJ, Caetano-Anollés G (2008c) Evolutionary patterns in the sequence and structure of transfer RNA: a window into early translation and the genetic code. PLoS One 3:e2799
Sun FJ, Caetano-Anollés G (2008d) Transfer RNA and the origins of diversified life. Sci Prog 91(Pt 3):265–284
Tanaka M, Ozawa T (1994) Strand asymmetry in human mitochondrial mutations. Genomics 22:27–335
Trifonov EN (2000) Consensus temporal order of amino acids and evolution of the triplet code. Gene 261:139–151
Van Waeg G, Niklasson F, de Verdier CH (1986) Deamination of guanine to xanthine: a metabolic pathway of underestimated importance in human purine catabolism? Adv Exp Med Biol 195A:425–430
Wan L, Kamba K, Nagata T, Katahira M (2019) An insight into the dependence of the deamination rate of human APOBEC3F on the length of single-stranded DNA, which is affected by the concentrations of APOBEC3F and single-stranded DNA. Biochim Biophys Acta Gen Subj S0304-4165(19):30088–30081
Wang S, Hu A (2016) Comparative study of spontaneous deamination of adenine and cytosine in unbuffered aqueous solution at room temperature. Chem Phys Lett 653:207–211
Warthi G, Seligmann H (2019) Transcripts with systematic nucleotide deletion of 1-12 nucleotide in human mitochondrion suggest potential non-canonical transcription. PLoS 14:e0217356
Wei SJ, Shi M, Chen XX, Sharkey MJ, van Achterberg C, Ye GY, He JH (2010) New views on strand asymmetry in insect mitochondrial genomes. PLoS One 5:e12708
Wickramasinghe NC, Wallis J, Wallis DH, Samaranayake A (2013) Fossil diatoms in a new carbonaceous meteorite. J Cosmol 21(37):14–38
Wolf YI, Koonin EV (2001) Origin of an animal mitochondrial DNA polymerase subunit via lineage-specific acquisition of a glycyl-tRNA synthetase from bacteria of the Thermus-Deinococcus group. Trends Genet 17:431–433
Xia X (2012) DNA replication and strand asymmetry in prokaryotic and mitochondrial genomes. Curr Genomics 13:16–27
Xia X (2018) Is there a mutation gradient along vertebrate mitochondrial genome mediated by genome replication? Mitochondrion, in press
Yarus M (2017) The genetic code and RNA-amino acid affinities. Life (Basel) 7:13
Yarus M, Christian EL (1989) Genetic code origins. Nature 342:349–350
Yarus M, Widmann JJ, Knight R (2009) RNA-amino acid binding: a stereochemical era for the genetic code. J Mol Evol 69:406–429
Zagrovic B, Bartonek L, Polyansky AA (2018) RNA-protein interactions in an unstructured context. FEBS Lett 592:2901–2916
Zheng Y, Lorenzo C, Beal PA (2017) DNA editing in DNA/RNA hybrids by adenosine deaminases that act on RNA. Nucleic Acids Res 45:3369–3377
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Matthias Waltert
This article is dedicated to the memory of Manfred Eigen, an immense pioneer in the field of theoretical biology of evolution.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Demongeot, J., Seligmann, H. Theoretical minimal RNA rings designed according to coding constraints mimic deamination gradients. Sci Nat 106, 44 (2019). https://doi.org/10.1007/s00114-019-1638-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-019-1638-5