Abstract
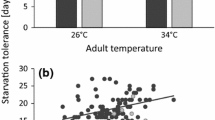
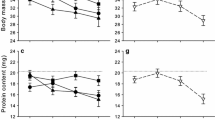
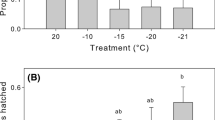
Predatory arthropods are used for biological control in greenhouses, but there is increasing interest to extend their use to the outdoor environment where temperatures are typically lower. Acclimation at low temperature increases the ability of ectotherms to cope with subsequent more extreme cold, but may involve costs or benefits to other performance traits. A recent study in mesostigmatid mites (Gaeolaelaps aculeifer) showed that starvation tolerance was improved following a period of cold exposure. However, the physiological mechanisms that underlie improved starvation tolerance following cold exposure were not investigated. To examine whether cold acclimation would also improve starvation tolerance in an insect, we repeated the starvation study in another arthropod predator, the pirate bug Orius majusculus, as well as in G. aculeifer. Before tests, the two species were acclimated at 10, 15, or 20 °C for 7 (G. aculeifer) or 16 (O. majusculus) days. We then analyzed the effects of thermal exposure on body composition, consumption, and basal metabolic rate in both species. Our results confirmed that exposure to low temperature improves starvation tolerance in these arthropod predators. Body composition analyses revealed that both species had accumulated larger lipid stores during exposure to colder temperature, which at least in part can explain the larger starvation tolerance following cold exposure. In contrast, consumption and basal metabolic rate were not changed by thermal acclimation. Our study indicates that predatory arthropods exposed to cold increase their physiological robustness and ability to endure environmental challenges, including low temperature and low prey availability.




Similar content being viewed by others
References
Addo-Bediako A, Chown S, Gaston K (2002) Metabolic cold adaptation in insects: a large-scale perspective. Funct Ecol 16:332–338
Alemu T, Alemneh T, Pertoldi C, Ambelu A, Bahrndorff S (2017) Costs and benefits of heat and cold hardening in a soil arthropod. Biol J Linn Soc 122:765–773
Alton LA, Condon C, White CR, Angilletta MJ (2017) Colder environments did not select for a faster metabolism during experimental evolution of Drosophila melanogaster. Evolution 71:145–152
Anderson JF (1970) Metabolic rates of spiders. Comp Biochem Physiol 33:51–72
Angilletta MJ (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Berrigan D (1997) Acclimation of metabolic rate in response to developmental temperature in Drosophila melanogaster. J Therm Biol 22:213–218
Bjørge JD, Overgaard J, Malte H, Gianotten N, Heckmann L-H (2018) Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J Insect Physiol 107:89–96
Bubliy OA, Kristensen TN, Kellermann V, Loeschcke V (2012) Plastic responses to four environmental stresses and cross-resistance in a laboratory population of Drosophila melanogaster. Funct Ecol 26:245–253
Chown SL, Gaston KJ (1999) Exploring links between physiology and ecology at macro-scales: the role of respiratory metabolism in insects. Biol Rev 74:87–120
Chown SL, Nicolson S (2004) Insect physiological ecology: mechanisms and patterns. Oxford University Press, Oxford
Chown SL, Terblanche JS (2007) Physiological diversity in insects: ecological and evolutionary contexts. Adv Insect Physiol 33:50–152
Colinet H, Boivin G (2011) Insect parasitoids cold storage: a comprehensive review of factors of variability and consequences. Biol Control 58:83–95
DeWitt TJ, Sih A, Wilson DS (1998) Costs and limits of phenotypic plasticity. Trends Ecol Evol 13:77–81
Dussutour A, Poissonnier L-A, Buhl J, Simpson SJ (2016) Resistance to nutritional stress in ants: when being fat is advantageous. J Exp Biol 219:824–833
Ghazy NA, Osakabe M, Negm MW, Schausberger P, Gotoh T, Amano H (2016) Phytoseiid mites under environmental stress. Biol Control 96:120–134
Hahn DA, Denlinger DL (2007) Meeting the energetic demands of insect diapause: nutrient storage and utilization. J Insect Physiol 53:760–773
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121
Harrison JF, Woods HA, Roberts SP (2012) Ecological and environmental physiology of insects. Oxford University Press, New York
Hart AJ, Bale JS, Tullett AG, Worland MR, Walters KFA (2002) Effects of temperature on the establishment potential of the predatory mite Amblyseius californicus McGregor (Acari: Phytoseiidae) in the UK. J Insect Physiol 48:593–599
Helgadóttir F, Toft S, Sigsgaard L (2017) Negative effects of low developmental temperatures on aphid predation by Orius majusculus (Heteroptera: Anthocoridae). Biol Control 114:59–64
Hodkinson I (2003) Metabolic cold adaptation in arthropods: a smaller-scale perspective. Funct Ecol 17:562–567
Hoffmann AA, Hallas R, Anderson AR, Telonis-Scott M (2005) Evidence for a robust sex-specific trade-off between cold tolerance and starvation tolerance in Drosophila melanogaster. J Evol Biol 18:804–810
Hoffmann AA, Sørensen JG, Loeschcke V (2003) Adaptation of Drosophila to temperature extremes: bringing together quantitative and molecular approaches. J Therm Biol 28:175–216
Jensen K, Kristensen TN, Overgaard J, Toft S, Sørensen JG, Holmstrup M (2017) Cold acclimation reduces predation rate and reproduction but increases cold- and starvation tolerance in the predatory mite Gaeolaelaps aculeifer Canestrini. Biol Control 114:150–157
Jensen K, Mayntz D, Wang T, Simpson SJ, Overgaard J (2010) Metabolic consequences of feeding and fasting on nutritionally different diets in the wolf spider Pardosa prativaga. J Insect Physiol 56:1095–1100
Jensen P, Overgaard J, Loeschcke V, Schou MF, Malte H, Kristensen TN (2014) Inbreeding effects on standard metabolic rate investigated at cold, benign and hot temperatures in Drosophila melanogaster. J Insect Physiol 62:11–20
Johnston IA, Bennett AF (1996) Animals and temperature: phenotypic and evolutionary adaptation. Cambridge University Press, Cambridge
Kristensen TN, Hoffmann AA, Overgaard J, Sørensen JG, Hallas R, Loeschcke V (2008) Costs and benefits of cold acclimation in field-released Drosophila. Proc Natl Acad Sci U S A 105:216–221
Kristensen TN, Kjeldal H, Schou MF, Nielsen JL (2016) Proteomic data reveal a physiological basis for costs and benefits associated with thermal acclimation. J Exp Biol 219:969–976
Lee KP, Jang T (2014) Exploring the nutritional basis of starvation resistance in Drosophila melanogaster. Funct Ecol 28:1144–1155
Luczynski A, Nyrop J, Shi A (2008) Pattern of female reproductive age classes in mass-reared populations of Phytoseiulus persimilis (Acari: Phytoseiidae) and its influence on population characteristics and quality of predators following cold storage. Biol Control 47:159–166
Messamah B, Kellermann V, Malte H, Loeschcke V, Overgaard J (2017) Metabolic cold adaptation contributes little to the interspecific variation in metabolic rates of 65 species of Drosophilidae. J Insect Physiol 98:309–316
Nyamukondiwa C, Terblanche JS (2009) Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): effects of age, gender and feeding status. J Therm Biol 34:406–414
Overgaard J, MacMillan HA (2017) The integrative physiology of insect chill tolerance. Annu Rev Physiol 79:8.1–8.22
Pijpe J, Brakefield PM, Zwaan BJ (2007) Phenotypic plasticity of starvation resistance in the butterfly Bicyclus anynana. Evol Ecol 21:589–600
Prosser CL (1955) Physiological variation in animals. Biol Rev 30:229–261
Scharf I, Wertheimer KO, Xin JL, Gilad T, Goldenberg I, Subach A (2017) Context-dependent effects of cold stress on behavioral, physiological, and life-history traits of the red flour beetle. Insect Sci in press. https://doi.org/10.1111/1744-7917.12497.
Scharf I, Wexler Y, MacMillan HA, Presman S, Simson E, Rosenstein S (2016) The negative effect of starvation and the positive effect of mild thermal stress on thermal tolerance of the red flour beetle, Tribolium castaneum. Sci Nat 103:20
Sinclair BJ, Marshall KE (2018) The many roles of fats in overwintering insects. J Exp Biol 221:jeb161836
Smith CR, Tschinkel WR (2009) Ant fat extraction with a Soxhlet extractor. Cold Spring Harb Protoc 4:832–835
Stamou GP, Asikidis MD, Argyropoulou MD, Iatrou GD (1995) Respiratory responses of oribatid mites to temperature changes. J Insect Physiol 41:229–233
Sørensen JG, Addison MF, Terblanche JS (2012) Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop Prot 38:87–94
Terblanche JS (2014) Physiological performance of field-released insects. Curr Oppin Insect Sci 4:60–66
Terblanche JS, Clusella-Trullas S, Deere JA, Van Vuuren BJ, Chown SL (2009) Directional evolution of the slope of the metabolic rate–temperature relationship is correlated with climate. Physiol Biochem Zool 82:495–503
Vreysen MJB, Robinson AS, Hendrichs J (2007) Area-wide control of insect pests: from research to field implementation. Springer, Dortrecht
Williams CM, Szejner-Sigal A, Morgan TJ, Edison AS, Allison DB, Hahn DA (2016) Adaptation to low temperature exposure increases metabolic rates independently of growth rates. Integr Comp Biol 56:62–72
Williams CM, Thomas RH, MacMillan HA, Marshall KE, Sinclair BJ (2011) Triacylglyceride measurement in small quantities of homogenised insect tissue: comparisons and caveats. J Insect Physiol 57:1602–1613
Acknowledgements
We thank Søren Toft and three anonymous reviewers for their constructive comments on earlier versions of the manuscript.
Funding
This study was funded by the Danish Council for Independent Research–Technology and Production Sciences (DFF-4184-00248).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by: Rumyana Jeleva
Rights and permissions
About this article
Cite this article
Jensen, K., Michaelsen, J.V., Larsen, M.T. et al. Increased lipid accumulation but not reduced metabolism explains improved starvation tolerance in cold-acclimated arthropod predators. Sci Nat 105, 65 (2018). https://doi.org/10.1007/s00114-018-1593-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00114-018-1593-6