Abstract
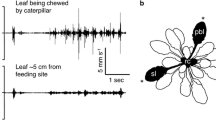
Finding and attracting mates can impose costs on males in terms of increased encounters with, and attraction of, predators. To decrease the likelihood of predation, males may modify mate-acquisition efforts in two main ways: they may reduce mate-searching efforts or they may reduce mate-attraction efforts. The specific behavior that males change in the presence of predator cues should depend upon the nature of risk imposed by the type of predator present in the environment. For example, sit-and-wait predators impose greater costs to males moving in search of mates. Here, we test whether cues of the presence of a sit-and-wait predator lead to a reduction in mate-searching but not mate-acquisition behavior. We used a member of the Enchenopa binotata complex of treehoppers—a clade of vibrationally communicating insects in which males fly in search of mates and produce mate-attraction signals when they land on plant stems. We tested for changes in mate-searching and signaling behaviors when silk from a web-building spider was present or absent. We found that males delayed flight when spider silk was present but only if they were actively searching for mates. These results suggest that males have been selected to reduce predation risk by adjusting how they move about their environment according to the cues of sit-and-wait predators.





Similar content being viewed by others
References
Bertram SM, Orozco SX, Bellani R (2004) Temporal shifts in conspicuousness: mate attraction displays of the Texas field cricket, Gryllus texensis. Ethology 110(12):963–975
Cocroft RB (2011) The public world of insect vibrational communication. Mol Ecol 20(10):2041–2043
Cocroft RB, Rodriguez RL (2005) The behavioral ecology of insect vibrational communication. Bioscience 55(4):323–334
Cocroft RB, Rodríguez RL, Hunt RE (2008) Host shifts, the evolution of communication and speciation in the Enchenopa binotata complex of treehoppers. In: Tilmon K (ed) Specialization, speciation, and radiation: the evolutionary biology of herbivorous insects. University of California Press, pp 88–100
Cocroft RB, Rodríguez RL, Hunt RE (2010) Host shifts and signal divergence: mating signals covary with host use in a complex of specialized plant-feeding insects. Biol J Linn Soc 99(1):60–72
Downes S (2002) Does responsiveness to predator scents affect lizard survivorship? Behav Ecol Sociobiol 52(1):38–42
Eiben B, Persons MH (2007) The effect of prior exposure to predator cues on chemically-mediated defensive behavior and survival in the wolf spider Rabidosa rabida (Araneae: Lycosidae). Behaviour 144:889–906
Fill A, Long EY, Finke DL (2012) Non-consumptive effects of a natural enemy on a non-prey herbivore population. Ecol Entomol 37:43–50
Foelix RF (1996) The biology of spiders. Oxford University Press, Inc., New York, USA
Fowler-Finn KD, Hebets EA (2011) The degree of response to increased predation risk corresponds to male secondary sexual traits. Behav Ecol 22(2):268–275
Garcia LV (2004) Escaping the Bonferroni iron claw in ecological studies. Oikos 105(3):657–663
Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct Ecol 21(3):394–407
Gomulkiewicz R, Kirkpatrick M (1992) Quantitative genetics and the evolution of reaction norms. Evolution 46(2):390–411
Hamilton KGA, Cocroft RB (2009) Establishing the identity of existing names in the North American Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae). Entomol News 120(5):554–565
Harwood JD, Sunderland KD, Symondson WOC (2003) Web-location by linyphiid spiders: prey-specific aggregation and foraging strategies. J Anim Ecol 72:745–756
Hawlena D, Stickland MS, Bradford MA, Schmitz OJ (2012) Fear of predation slows plant-letter decomposition. Science 336:1434–1438
Hedrick AV (2000) Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc Roy Soc Lond Ser B-Biol Sci 267(1444):671–675
Helfman GS (1989) Threat-sensitive predator avoidance in damselfish–trumpetfish interactions. Behav Ecol Sociobiol 24:47–58
Hlivko JT, Rypstra AL (2003) Spiders reduce herbivory: nonlethal effects of spiders on the consumption of soybean leaves by beetle pests. Ann Entomol Soc Am 96:914–919
Kortet R, Hedrick A (2004) Detection of the spider predator, Hololena nedra by naive juvenile field crickets (Gryllus integer) using indirect cues. Behaviour 141:1189–1196
Krupa JJ, Sih A (1998) Fishing spiders, green sunfish, and a stream-dwelling water strider: male–female conflict and prey responses to single versus multiple predator environments. Oecologia 117(1–2):258–265
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Can J Zool-Rev Can Zool 68(4):619–640
Magnhagen C (1991) Predation risk as a cost of reproduction. Trends Ecol Evol 6:183–186
Makin DF, Payne HFP, Kerley GIH, Shrader AM (2012) Foraging in a 3-D world: how does predation risk affect space use of vervet monkeys? J Mammal 93(2):422–428
McGhee KE, Pintor LM, Bell AM (2013) Reciptrocal behavioral plasticity and behavioral types during predatory-prey interactions. Am Nat 182(6):704–717
Milinski M, Heller R (1978) Influence of a predator on the optimal foraging behaviour of sticklebacks (Gasterosteus aculeatus L.). Nature 275:642–644
Moore SD (1987) Male-biased mortality in the butterfly Euphydryas editha: a novel cost of mate acquisition. Am Nat 130:306–309
Moran NA (1992) The evolutionary maintenance of alternative phenotypes. Am Nat 139(5):971–989
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100(2):403–405
Nakagawa S (2004) A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15(6):1044–1045
Persons MH, Walker SE, Rypstra AL, Marshall SD (2001) Wolf spider predator avoidance tactics and survival in the presence of diet-associated predator cues (Araneae: Lycosidae). Anim Behav 61:43–51
Persons MH, Walker SE, Rypstra AL (2002) Fitness costs and benefits of antipredator behavior mediated by chemotactile cues in the wolf spider Pardosa milvina (Araneae: Lycosidae). Behav Ecol 13(3):386–392
Relyea RA (2001) The relationship between predation risk and antipredator responses in larval anurans. Ecology 82(2):541–554
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rodríguez RL, Cocroft RB (2006) Divergence in female duetting signals in the Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae). Ethology 112(12):1231–1238
Rodríguez RL, Sullivan LE, Cocroft RB (2004) Vibrational communication and reproductive isolation in the Enchenopa binotata species complex of treehoppers (Hemiptera: Membracidae). Evolution 58(3):571–578
Rodríguez RL, Ramaswamy K, Cocroft RB (2006) Evidence that female preferences have shaped male signal evolution in a clade of specialized plant-feeding insects. Proc Roy Soc B-Biol Sci 273(1601):2585–2593
Rodríguez RL, Cocroft RB, Haen C, Fowler-Finn KD (2012) Males adjust signaling effort based on cues arising from the expression of female mate preferences. Behav Ecol 23(6):1218–1225
Rypstra AL, Buddle CM (2013) Spider silk reduces insect herbivory. Biol Lett 9(1):20120948
Schmidt-Entling MH, Siegenthaler E (2009) Herbivore release through cascading risk effects. Biol Lett 6:773–776
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–163
Sih A (1980) Optimal behavior: can foragers balance two conflicting demands? Science 210:1041–1043
Sih A (1987) Predators and prey lifestyles: an evolutionary and ecological overview. In: Kerfoot WC, Sih A (eds) Predation: direct and indirect impacts on aquatic communities. University Press of New England, Hanover, New Hampshire, USA
Simon VB (2007) Not all signals are equal: male brown anole lizards (Anolis sagrei) selectively decrease pushup frequency following a simulated predatory attack. Ethology 113(8):793–801
Taylor AR, Persons MH, Rypstra AL (2005) The effect of perceived predation risk on male courtship and copulatory behavior in the wolf spider Pardosa milvina (Araneae, Lycosidae). J Arachnol 33(1):76–81
Templeton CN, Shriner WM (2004) Multiple selection pressures influence Trinidadian guppy (Poecilia reticulata) antipredator behavior. Behav Ecol 15(4):673–678
Via S, Gomulkiewicz R, Dejong G, Scheine SM, Schlichting CD, VanTiendern PH (1995) Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol Evol 10:212–217
Virant-Doberlet M, King RA, Polajnar J, Symondson WOC (2011) Molecular diagnostics reveal spiders that exploit prey vibrational signals used in sexual communication. Mol Ecol 20(10):2204–2216
Zuk M, Kolluru GR (1998) Exploitation of sexual signals by predators and parasitoids. Q Rev Biol 73(4):415–438
Acknowledgments
The authors thank T. Schuck and C. Haen for help with insect and plant care. This work was funded by National Science Foundation Grant IOS-0919962 to RLR, National Science Foundation Grant IOS-1120790 to RLR and KDFF, and SURF funding from the University of Wisconsin-Milwaukee to NA-W and MA-W.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Fowler-Finn, K.D., Al-Wathiqui, N., Cruz, D. et al. Male Enchenopa treehoppers (Hemiptera: Membracidae) vary mate-searching behavior but not signaling behavior in response to spider silk. Naturwissenschaften 101, 211–220 (2014). https://doi.org/10.1007/s00114-014-1145-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-014-1145-7