Abstract
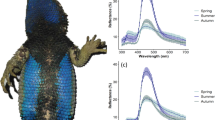
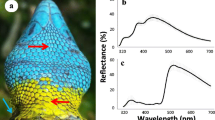
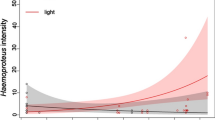
During female mate choice, conspicuous male sexual signals are used to infer male quality and choose the best sire for the offspring. The theory of parasite-mediated sexual selection (Hamilton–Zuk hypothesis) presumes that parasite infection can influence the elaboration of sexual signals: resistant individuals can invest more energy into signal expression and thus advertise their individual quality through signal intensity. By preferring these males, females can provide resistance genes for their offspring. Previous research showed that nuptial throat colour of male European green lizard, Lacerta viridis, plays a role in both inter- and intrasexual selections as a condition-dependent multiple signalling system. The aim of this study was to test the predictions of the Hamilton–Zuk hypothesis on male European green lizards. By blood sampling 30 adult males during the reproductive season, we found members of the Haemogregarinidae family in all but one individual (prevalence = 96 %). The infection intensity showed strong negative correlation with the throat and belly colour brightness in line with the predictions of the Hamilton–Zuk hypothesis. In addition, we found other correlations between infection intensity and other fitness-related traits, suggesting that parasite load has a remarkable effect on individual fitness. This study shows that throat patch colour of the European green lizards not only is a multiple signalling system but also possibly acts as an honest sexual signal of health state in accordance with the Hamilton–Zuk hypothesis.


Similar content being viewed by others
References
Al-Ghamdy AO (2011) A light microscopic study on the haemogregarine species infecting the lizard Acanthodactylus schmidti from Saudi Arabia. J Egypt Soc Parasitol 41(1):7–15
Amo L, Lopez P, Martin J (2005) Prevalence and intensity of haemogregarine blood parasites and their mite vectors in the common wall lizard, Podarcis muralis. Parasitol Res 96(6):378–381
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Bajer K, Molnar O, Torok J, Herczeg G (2010) Female European green lizards (Lacerta viridis) prefer males with high ultraviolet throat reflectance. Behav Ecol Sociobiol 64(12):2007–2014
Bajer K, Molnar O, Torok J, Herczeg G (2011) Ultraviolet nuptial colour determines fight success in male European green lizards (Lacerta viridis). Biol Lett 7(6):866–868. doi:10.1098/rsbl.2011.0520
Bajer K, Molnar O, Torok J, Herczeg G (2012) Temperature, but not available energy, affects the expression of a sexually selected ultraviolet (UV) colour trait in male European green lizards. PLoS ONE 7 (3):e34359. DOI 10.1371/journal.pone.0034359
Barnard SM, Upton SJ (1994) A veterinary guide to the parasites of reptiles, vol. 1. Protozoa. Krieger, Malabar
Borgia G (1986) Satin bowerbird parasites—a test of the bright male hypothesis. Behav Ecol Sociobiol 19:355–358
Borgia G, Collis K (1990) Parasites and bright male plumage in the satin bowerbird (Ptilonorhynchus violaceus). Am Zool 30:279–285
Boulinier T, Sorci G, Monnat JY, Danchin E (1997) Parent–offspring regression suggests heritable susceptibility to ectoparasites in a natural population of kittiwake Rissa tridactyla. J Evol Biol 10(1):77–85
Bouma MJ, Smallridge CJ, Bull CM, Komdeur J (2007) Susceptibility to infection by a haemogregarine parasite and the impact of infection in the Australian sleepy lizard Tiliqua rugosa. Parasitol Res 100(5):949–954
Brawner WR, Hill GE, Sundermann CA (2000) Effects of coccidial and mycoplasmal infections on carotenoid-based plumage pigmentation in male house finches. Auk 117:952–963
Brown GP, Shilton CM, Shine R (2006) Do parasites matter? Assessing the fitness consequences of haemogregarine infection in snakes. Can J Zool 84(5):668–676
Budden AE, Dickinson JL (2009) Signals of quality and age: the information content of multiple plumage ornaments in male western bluebirds Sialia mexicana. J Avian Biol 40(1):18–27. doi:10.1111/j.1600-048X.2008.04344.x
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78(4):575–595
Charge R, Saint Jalme M, Lacroix F, Cadet A, Sorci G (2010) Male health status, signalled by courtship display, reveals ejaculate quality and hatching success in a lekking species. J Anim Ecol 79(4):843–850
Clayton DH (1990) Mate choice in experimentally parasitized rock doves—lousy males lose. Am Zool 30(2):251–262
Clayton DH (1991) The influence of parasites on host sexual selection. Par Tod 7:329–334
Davies AJ, Reed CC, Smit NJ (2003) An unusual intraerythrocytic parasite of Parablennius cornutus from South Africa. J Parasitol 89(5):913–917
del Cerro S, Merino S, Martinez-de la Puente J, Lobato E, Ruiz-de Castañeda R, Rivero-de Aaguilar J, Martinez J, Morales J, Tomas G, Moreno J (2010) Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caeruleus). Oecologia 162:825–835
Dunlap KD (1993) Effects of nymphal ticks and their interaction with malaria on the physiology of male fence lizards. Copeia 4:1045–1048
Garcia-Ramirez A, Delgado-Garcia JD, Foronda-Rodriguez P, Abreu-Acosta N (2005) Haematozoans, mites and body condition in the oceanic island lizard Gallotia atlantica (Peters and Doria, 1882) (Reptilia: Lacertidae). J Nat Hist 39(17):1299–1305
Grether GF, Kolluru GR, Nersissian K (2004) Individual colour patches as multicomponent signals. Biol Rev 79:583–610. doi:10.1017/S1464793103006390
Hagelin JC (2002) The kinds of traits involved in male–male competition: a comparison of plumage, behavior, and body size in quail. Behav Ecol 13:384–387
Hamilton PS, Sullivan BK (2005) Female mate attraction in ornate tree lizards, Urosaurus ornatus: a multivariate analysis. Anim Behav 69:219–224. doi:10.1016/j.anbehav.2004.03.011
Hamilton WD, Zuk M (1982) Heritable true fitness and bright birds—a role for parasites. Science 218(4570):384–387
Healey M, Uller T, Olsson M (2007) Seeing red: morph-specific contest success and survival rates in a colour-polymorphic agamid lizard. Anim Behav 74:337–341
Hussein ANA (2006) Light and transmission electron microscopic studies of a haemogregarine in naturally infected fan-footed gecko (Ptyodactylus hasselquistii). Parasitol Res 98(5):468–471. doi:10.1007/s00436-005-0084-9
Huyghe K, Herrel A, Adriaens D, Tadic Z, Van Damme R (2009) It is all in the head: morphological basis for differences in bite force among colour morphs of the Dalmatian wall lizard. Biol J Linn Soc 96(1):13–22. doi:10.1111/j.1095-8312.2008.01103.x
Lainson R, De Souza MC, Franco CM (2007) Natural and experimental infection of the lizard Ameiva ameiva with Hemolivia stellata (Adeleina : Haemogregarinidae) of the toad Bufo marinus. Parasite-Journal De La Societe Francaise De Parasitologie 14(4):323–328
Lebas NR, Marshall NJ (2001) No evidence of female choice for a condition-dependent trait in the agamid lizard, Ctenophorus ornatus. Behaviour 138:965–980
Lefcort H, Blaustein AR (1991) Parasite load and brightness in lizards—an interspecific test of the Hamilton and Zuk hypothesis. J Zool 224:491–499
Leu ST, Kappeler PM, Bull CM (2010) Refuge sharing network predicts ectoparasite load in a lizard. Behav Ecol Sociobiol 64(9):1495–1503
Martin J, Amo L, Lopez P (2008) Parasites and health affect multiple sexual signals in male common wall lizards, Podarcis muralis. Naturwissenschaften 95(4):293–300. doi:10.1007/s00114-007-0328-x
Martin J, Civantos E, Amo L, Lopez P (2007) Chemical ornaments of male lizards Psammodromus algirus may reveal their parasite load and health state to females. Behav Ecol Sociobiol 62(2):173–179. doi:10.1007/s00265-007-0451-x
Martin J, Lopez P (2009) Multiple color signals may reveal multiple messages in male Schreiber's green lizards, Lacerta schreiberi. Behav Ecol Sociobiol 63(12):1743–1755
McGraw KJ, Hill GE (2000) Differential effects of endoparasitism on the expression of carotenoid- and melanin-based ornamental coloration. Proc R Soc B Biol Sci 267(1452):1525–1531
McGraw KJ, Mackillop EA, Dale J, Hauber ME (2002) Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J Exp Biol 205(23):3747–3755
Moller AP (1990) Parasites and sexual selection—current status of the Hamilton and Zuk hypothesis. J Evol Biol 3(5–6):319–328
Moller AP (1994) Directional selection on directional asymmetry: testes size and secondary sexual characters in birds. Proc R Soc B Biol Sci 258(1352):147–151. doi:10.1098/rspb.1994.0155
Moller AP, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132(4):492–500. doi:10.1007/s00442-002-0952-2
Molnar O, Bajer K, Torok J, Herczeg G (2012) Individual quality and nuptial throat colour in male European green lizards. J Zool 287(4):233–239. doi:10.1111/j.1469-7998.2012.00916.x
Murtaugh PA (2009) Performance of several variable-selection methods applied to real ecological data. Ecol Lett 12(10):1061–1068
Olsson M, Wapstra E, Madsen T, Ujvari B, Rugfelt C (2005) Costly parasite resistance: a genotype-dependent handicap in sand lizards? Biol Lett 1(3):375–377. doi:10.1098/rsbl.2005.0339
Oppliger A, Celerier ML, Clobert J (1996) Physiological and behaviour changes in common lizards parasitized by haemogregarines. Parasitology 113:433–438
Palacios MG, Winkler DW, Klasing KC, Hasselquist D, Vleck CM (2011) Consequences of immune system aging in nature: a study of immunosenescence costs in free-living tree swallows. Ecology 92(4):952–966
Palmer AR, Strobeck C (1986) Fluctuating asymmetry—measurement, analysis, patterns. Annu Rev Ecol Syst 17:391–421
Paperna I, Lainson R (2004) Hepatozoon cf. terzii (Sambon & Seligman, 1907) infection in the snake boa constrictor constrictor from north Brazil: transmission to the mosquito Culex quinquefasciatus and the lizard Tropidurus torquatus. Parasite-Journal De La Societe Francaise De Parasitologie 11(2):175–181
Peters A, Delhey K, Johnsen A, Kempenaers B (2007) The condition-dependent development of carotenoid-based and structural plumage in nestling blue tits: males and females differ. Am Nat 169:122–136
Petit G, Landau I, Baccam D, Lainson R (1990) Description et cycle biologique d’Hemolivia stellata n. g., n. sp., hémogrégarine de crapauds brésiliens. Ann Parasitol Hum Comp 65:3–15
Ressel S, Schall JJ (1989) Parasites and showy males—malarial infection and color variation in fence lizards. Oecologia 78(2):158–164. doi:10.1007/Bf00377151
Roca V, Galdon MA (2010) Haemogregarine blood parasites in the lizards Podarcis bocagei (Seoane) and P. carbonelli (Perez-Mellado) (Sauria: Lacertidae) from NW Portugal. Syst Parasitol 75:75–79
Saks L, Ots I, Horak P (2003) Carotenoid-based plumage coloration of male greenfinches reflects health and immunocompetence. Oecologia 134(3):301–307. doi:10.1007/s00442-002-1125-z
Schall JJ, Dearing MD (1987) Malarial parasitism and male competition for mates in the western fence lizard, Sceloporus occidentalis. Oecologia 73(3):389–392. doi:10.1007/Bf00385255
Schall JJ, Staats CM (1997) Parasites and the evolution of extravagant male characters: Anolis lizards on Caribbean islands as a test of the Hamilton–Zuk hypothesis. Oecologia 111(4):543–548
Sinervo B, Lively CM (1996) The rock-paper-scissors game and the evolution of alternative male strategies. Nature 380(6571):240–243. doi:10.1038/380240a0
Smallridge CJ, Bull CM (1999) Transmission of the blood parasite Hemolivia mariae between its lizard and tick hosts. Parasitol Res 85(10):858–863
Stapley J, Whiting MJ (2006) Ultraviolet signals fighting ability in a lizard. Biol Lett 2(2):169–172. doi:10.1098/rsbl.2005.0419
Telford SR (2009) Haemoparasites of the Reptilia. CRC, Boca Raton
Tripet F, Richner H (1999) Density-dependent processes in the population dynamics of a bird ectoparasite Ceratophyllus gallinae. Ecology 80(4):1267–1277. doi:10.1890/0012-9658(1999)080[1267:Ddpitp]2.0.Co;2
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man, 1871–197. Aldine, Chicago, pp 136–179
van Valen L (1962) A study of fluctuating asymmetry. Evolution 16:125–142
Weiss SL (2006) Female-specific color is a signal of quality in the striped plateau lizard (Sceloporus virgatus). Behav Ecol 17(5):726–732
Whiting MJ, Stuart-Fox DM, O'Connor D, Firth D, Bennett NC, Blomberg SP (2006) Ultraviolet signals ultra-aggression in a lizard. Anim Behav 72:353–363
Wikel SK, Ramachandra RN, Bergman DK, Burkot TR, Piesman J (1997) Infestation with pathogen-free nymphs of the tick Ixodes scapularis induces host resistance to transmission of Borrelia burgdorferi by ticks. Infect Immun 65:335–338
Zahavi A (1975) Mate selection—selection for a handicap. J Theor Biol 53:205–214
Zahavi A (1977) Cost of honesty—(further remarks on handicap principle). J Theor Biol 67(3):603–605
Acknowledgments
We would like to thank Prof. Joseph J. Schall for his indispensable help in identifying the blood parasites. We also thank Michael L. Logan for his useful comments and correcting the English. The study was supported by OTKA (Hungarian Scientific Research Fund, ref. no. F68403 and K105517). We thank Middle–Danube–Valley Environmental, Nature and Water Inspectorate for the permission to conduct this study (project no. 31203-3/2010).
Ethical standards
Experiments were performed according to the guidelines of the Hungarian Act of Animal Care and Experimentation (1998, XXVIII, section 243/ 1998), which conforms to the regulation of animal experiments by the European Union.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: Sven Thatje
Rights and permissions
About this article
Cite this article
Molnár, O., Bajer, K., Mészáros, B. et al. Negative correlation between nuptial throat colour and blood parasite load in male European green lizards supports the Hamilton–Zuk hypothesis. Naturwissenschaften 100, 551–558 (2013). https://doi.org/10.1007/s00114-013-1051-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-013-1051-4