Abstract
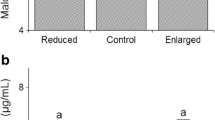
Many organisms show differences between males and females in growth rate and crucial life history parameters, such as longevity. Considering this, we may expect levels of toxic metabolic by-products of the respiratory chain, such as reactive oxygen species (ROS), to vary with age and sex. Here, we analyse ROS levels in female Australian painted dragon lizards (Ctenophorus pictus) and their offspring using fluorescent probes and flow cytometry. Basal level of four ROS species (singlet oxygen, peroxynitrite, superoxide and H2O2) measured with a combined marker, and superoxide measured specifically, varied significantly among families but not between the sexes. When blood cells from offspring were chemically encouraged to accelerate the electron transport chain by mitochondrial uncoupling, net superoxide levels were three times higher in daughters than sons (resulting in levels outside of the normal ROS range) and varied among mothers depending on offspring sex (significant interaction between maternal identity and offspring sex). In offspring, there were depressive effects on ROS of size-controlled relative clutch size, which relies directly on circulating levels of vitellogenin, a confirmed antioxidant in some species. Thus, levels of reactive oxygen species varies among females, offspring and in relation to reproductive investment in a manner that makes its regulatory processes likely targets of selection.

Similar content being viewed by others
References
Barja G (2004) Ageing in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism. Biol Rev 79:235–251
Birky CW (1994) Uniparental inheritance and mitochondrial and chloroplast genes: mechanisms and evolution. Proc Natl Acad Sci USA 92:11331–11338
Constantini D, Dell’Omo G (2006) Environmental and genetic components of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:575–579
Constantini D, Casagrande S, De Filipis S, Brambilla G, Fanfani A, Tagliavini J, Dell’Omo G (2006) Correlates of oxidative stress in wild kestrel nestlings (Falco tinnunculus). J Comp Physiol B 176:329–337
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 400:239–247
Gartska WR, Camazine B, Crews D (1982) Interactions of behavior and physiology during the annual reproductive cycle of the red-sided garter snake (Thamnophis sirtalis parietalis). Herpetologia 38(1):104–123
Halliwell B, Gutteridge JM (2002) Free radicals in biology and medicine, 3rd edn. Oxford University Press. Thomson Press, India, pp 796–799
Harman D (1957) Ageing: a theory based on free radical and radiation chemistry. J Gerontol 2:298–300
Henderson LM, Chappell JB, Jones OT (1988) Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J 255:285–290
Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C (2003) Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424:277–284
Olsson M, Shine R (2001) Facultative sex allocation in snow skink lizards (Niveoscincus microlepidotus). J Evol Biol 14:120–128
Partridge L, Gems D, Withers DJ (2005) Sex and death: what is the connection. Cell 120:461–472
Seehuus S-C, Norberg K, Gimsa U, Krekling T, Amdam G (2006) Reproductive protein protects functionally sterile honey bee workers from oxidative stress. Proc Natl Acad Sci U S A 103:962–967
Shigenaga MK, Hagen TM, Ames BN (1994) Oxidative damage and mitochondrial decay in ageing. Proc Natl Acad Sci U S A 91:10771–10778
Spence M (2005) The handbook. A guide to fluorescent probes and labeling technologies, 10th edn. Invitrogen Molecular Probes, USA
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Venditti P, Piro MC, Axtiaco G, DiMeo S (1996) Effects of exercise on tissue antioxidant capacity and heart electrical potential after antioxidant supplementation in endurance athletes. Am J Clin Nutr 65:1052–1056
Vowells S, Sekhsaria S, Malech H, Shalit M, Fleisher T (1995) Flow cytometric analysis of the granulocyte respiratory burst: a comparison study of fluorescent probes. J Immun Meth 178:89–97
Wallace DC, Lott MT (2002) Mitochondrial defects of common diseases. In: King RA, Rotter JI, Motulsky AG (eds) The genetic basis of common diseases. 2nd edn. Oxford University Press, New York
Acknowledgements
The Australian Research Council (M.O.) and Wenner-Gren Foundations (T.U.) provided financial support. We thank E. and G. Snaith for logistic support. We thank three anonymous reviewers for their input and helpful suggestions, which improved the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Olsson, M., Wilson, M., Uller, T. et al. Variation in levels of reactive oxygen species is explained by maternal identity, sex and body-size-corrected clutch size in a lizard. Naturwissenschaften 96, 25–29 (2009). https://doi.org/10.1007/s00114-008-0444-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-008-0444-2