Abstract
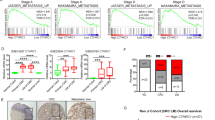
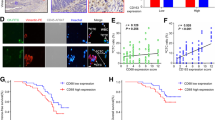
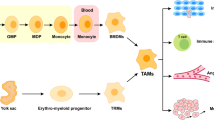
Tumor-associated macrophages (TAMs) represent a key factor in the tumor immune microenvironment (TME), exerting significant influence over tumor migration, invasion, immunosuppressive features, and drug resistance. Collagen triple helix repeat containing 1 (CTHRC1), a 30 KDa protein which was secreted during the tissue-repair process, is highly expressed in several malignant tumors, including colorectal cancer (CRC). Previous studies demonstrated that CTHRC1 expression in TAMs was positively correlated to M2 macrophage polarization and liver metastasis, while our discovery suggesting a novel mechanism that CTHRC1 secreted from cancer cell could indirectly interplay with TAMs. In this study, the high expression level of CTHRC1 was evaluated in CRC based on GEO and TCGA databases. Further, CTHRC1 was detected high in all stages of CRC patients by ELISA and was correlated to poor prognosis. Multispectral imaging of IHC demonstrated that M2 macrophage infiltration was increased accompanied with CTHRC1 enrichment, suggesting that CTHRC1 may have chemotactic effect on macrophages. In vitro, CTHRC1 could have chemotactic ability of macrophage in the presence of HT-29 cell line. Cytokine microarray revealed that CTHRC1 could up-regulate the CCL15 level of HT-29, pathway analysis demonstrated that CTHRC1 could regulate CCL15 by controlling the TGFβ activation and Smad phosphorylation level. In vivo, knocking down of CTHRC1 from CT-26 also inhibits tumor formation. In conclusion, CTHRC1 could promote the chemotactic ability of macrophages by up-regulating CCL15 via TGFβ/Smad pathway; additionally, a high level of CTHRC1 could promote macrophage’s M2 polarization. This discovery may be related to tumor immune tolerance and tumor immunotherapy resistance in CRC.
Key messages
-
CTHRC1 promotes CRC progression by up-regulating CCL15 via TGF-β/Smad pathways to further recruit tumor-associated macrophages.
-
By the means of autocrine or paracrine, CTHRC1 can indeed promote macrophage chemotaxis and enhance the infiltration of macrophages in tumor tissues but in the presence of tumor cells.
-
CAFs were another source of CTHRC1, indicating CTHRC1 can infiltrate tumor islet as well as the stomal and be secreted from both tumor cells and CAFs.
-
This study validated CTHRC1 as a potential immune therapy target CRC.






Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
References
Siegel RL, Miller KD, Fedewa SA et al (2017) Colorectal cancer statistics, 2017. CA: Cancer J Clin 67(3):177–193
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin 71(3):209–249
Segnan N, Armaroli P (2017) Early detection versus prevention in colorectal cancer screening: Methods estimates and public health implications. Cancer 123(24):4767–4769
Marcuello M, Vymetalkova V, Neves RPL et al (2019) Circulating biomarkers for early detection and clinical management of colorectal cancer. Mol Aspects Med 69:107–122
Miller KD, Nogueira L, Devasia T et al (2022) Cancer treatment and survivorship statistics, 2022. CA: Cancer J Clin 72(5):409–436
Li N, Lu B, Luo C et al (2021) Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett 522:255–268
Noy R, Pollard JW (2014) Tumor-associated macrophages: from mechanisms to therapy. Immunity 41(1):49–61
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L (2019) Macrophages and metabolism in the tumor microenvironment. Cell Metab 30(1):36–50
Chen D, Zhang X, Li Z, Zhu B (2021) Metabolic regulatory crosstalk between tumor microenvironment and tumor-associated macrophages. Theranostics 11(3):1016–1030
Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. International journal of molecular sciences. Jun 29 2021;22(13).
Han S, Wang W, Wang S et al (2021) Tumor microenvironment remodeling and tumor therapy based on M2-like tumor associated macrophage-targeting nano-complexes. Theranostics 11(6):2892–2916
Pan Y, Yu Y, Wang X, Zhang T (2020) Tumor-associated macrophages in tumor immunity. Front Immunol 11:583084
Lonardi S, Scutera S, Licini S et al (2020) CSF1R is required for differentiation and migration of Langerhans cells and Langerhans cell histiocytosis. Cancer Immunol Res 8(6):829–841
Rao R, Han R, Ogurek S et al (2022) Glioblastoma genetic drivers dictate the function of tumor-associated macrophages/microglia and responses to CSF1R inhibition. Neuro Oncol 24(4):584–597
Ji P, Gong Y, Jin ML et al (2022) In vivo multidimensional CRISPR screens identify Lgals2 as an immunotherapy target in triple-negative breast cancer. Sci Adv 8(26):eabl8247
Min AKT, Mimura K, Nakajima S et al (2021) Therapeutic potential of anti-VEGF receptor 2 therapy targeting for M2-tumor-associated macrophages in colorectal cancer. Cancer Immunol, Immunother : CII 70(2):289–298
Porta C, Ippolito A, Consonni FM et al (2018) Protumor steering of cancer inflammation by p50 NF-κB enhances colorectal cancer progression. Cancer Immunol Res 6(5):578–593
Biller LH, Schrag D (2021) Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 325(7):669–685
Ding X, Huang R, Zhong Y et al (2020) CTHRC1 promotes gastric cancer metastasis via HIF-1α/CXCR4 signaling pathway. Biomed Pharmacother 123:109742
Kim JH, Baek TH, Yim HS et al (2013) Collagen triple helix repeat containing-1 (CTHRC1) expression in invasive ductal carcinoma of the breast: the impact on prognosis and correlation to clinicopathologic features. Pathol Oncol Res : POR 19(4):731–737
Li J, Wang Y, Ma M et al (2019) Autocrine CTHRC1 activates hepatic stellate cells and promotes liver fibrosis by activating TGF-β signaling. EBioMedicine 40:43–55
Zhang XL, Hu LP, Yang Q et al (2021) CTHRC1 promotes liver metastasis by reshaping infiltrated macrophages through physical interactions with TGF-β receptors in colorectal cancer. Oncogene 40(23):3959–3973
Jiang N, Cui Y, Liu J et al (2016) Multidimensional roles of collagen triple helix repeat containing 1 (CTHRC1) in malignant cancers. J Cancer 7(15):2213–2220
LeClair R, Lindner V (2007) The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor, beta signaling. Trends Cardiovasc Med 17(6):202–205
Qin S, Zheng JH, Xia ZH, Qian J, Deng CL, Yang SL (2019) CTHRC1 promotes wound repair by increasing M2 macrophages via regulating the TGF-β and notch pathways. Biomed Pharmacother 113:108594
Mei D, Zhu Y, Zhang L, Wei W (2020) The role of CTHRC1 in regulation of multiple signaling and tumor progression and metastasis. Mediators Inflamm
Wong HY, Sheng Q, Hesterberg AB et al (2022) Single cell analysis of cribriform prostate cancer reveals cell intrinsic and tumor microenvironmental pathways of aggressive disease. Nat Commun 13(1):6036
Ni S, Ren F, Xu M et al (2018) CTHRC1 overexpression predicts poor survival and enhances epithelial-mesenchymal transition in colorectal cancer. Cancer Med 7(11):5643–5654
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425(6958):577–584
Zheng L, Liang H, Zhang Q et al (2022) circPTEN1, a circular RNA generated from PTEN, suppresses cancer progression through inhibition of TGF-β/Smad signaling. Mol Cancer 21(1):41
Wang X, Lai Q, He J et al (2019) LncRNA SNHG6 promotes proliferation, invasion and migration in colorectal cancer cells by activating TGF-β/Smad signaling pathway via targeting UPF1 and inducing EMT via regulation of ZEB1. Int J Med Sci 16(1):51–59
Guo Z, Zhang X, Zhu H et al (2021) TELO2 induced progression of colorectal cancer by binding with RICTOR through mTORC2. Oncol Rep 45(2):523–534
Cassetta L, Pollard JW (2018) Targeting macrophages: therapeutic approaches in cancer. Nature reviews. Drug Discov 17(12):887–904
Jaynes JM, Sable R, Ronzetti M et al (2020) Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci Transl Med 12(530)
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23(11):549–555
Sial N, Ahmad M, Hussain MS et al (2021) CTHRC1 expression is a novel shared diagnostic and prognostic biomarker of survival in six different human cancer subtypes. Sci Rep 11(1):19873
Qin R, Ren W, Ya G et al (2022) Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med
Yin X, Han S, Song C et al (2019) Metformin enhances gefitinib efficacy by interfering with interactions between tumor-associated macrophages and head and neck squamous cell carcinoma cells. Cell Oncol (Dordr) 42(4):459–475
Guo T, Hajdu M, Agaram NP et al (2009) Mechanisms of sunitinib resistance in gastrointestinal stromal tumors harboring KITAY502-3ins mutation: an in vitro mutagenesis screen for drug resistance. Clin Cancer Res 15(22):6862–6870
Hammond WA, Swaika A, Mody K (2016) Pharmacologic resistance in colorectal cancer: a review. Therapeutic Advances in Medical Oncology 8(1):57–84
Woolston A, Khan K, Spain G et al (2019) Genomic and transcriptomic determinants of therapy resistance and immune landscape evolution during anti-EGFR treatment in colorectal cancer. Cancer Cell 36(1):35–50.e39
Zhao L, Wang W, Niu P, Luan X, Zhao D, Chen Y (2022) The molecular mechanisms of CTHRC1 in gastric cancer by integrating TCGA, GEO and GSA datasets. Front Genet 13:900124
DeNardo DG, Ruffell B (2019) Macrophages as regulators of tumour immunity and immunotherapy. Nature reviews. Immunology 19(6):369–382
Timperi E, Gueguen P, Molgora M et al (2022) Lipid-associated macrophages are induced by cancer-associated fibroblasts and mediate immune suppression in breast cancer. Can Res 82(18):3291–3306
Mao X, Xu J, Wang W et al (2021) Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer 20(1):131
Tang PC, Chung JY, Xue VW et al (2022) Smad3 promotes cancer-associated fibroblasts generation via macrophage-myofibroblast transition. Adv Sci (Weinheim, Baden-Wurttemberg, Germany) 9(1):e2101235
Kobayashi H, Gieniec KA, Lannagan TRM et al (2022) The origin and contribution of cancer-associated fibroblasts in colorectal carcinogenesis. Gastroenterology 162(3):890–906
Liu LZ, Zhang Z, Zheng BH et al (2019) CCL15 Recruits suppressive monocytes to facilitate immune escape and disease progression in hepatocellular carcinoma. Hepatology (Baltimore, Md.) 69(1):143–159
Itatani Y, Kawada K, Fujishita T et al (2013) Loss of SMAD4 from colorectal cancer cells promotes CCL15 expression to recruit CCR1+ myeloid cells and facilitate liver metastasis. Gastroenterology 145(5):1064–1075.e1011
Liang Q, Tang C, Tang M, Zhang Q, Gao Y, Ge Z (2019) TRIM47 is up-regulated in colorectal cancer, promoting ubiquitination and degradation of SMAD4. J Exp Clin Cancer Res: CR 38(1):159
Inamoto S, Itatani Y, Yamamoto T et al (2016) Loss of SMAD4 promotes colorectal cancer progression by accumulation of myeloid-derived suppressor cells through the CCL15-CCR1 chemokine axis. Clin Cancer Res : Offic J Am Assoc Cancer Res 22(2):492–501
Shen R, Cai X, Shen D et al (2022) Long noncoding RNA LINC00518 contributes to proliferation and metastasis in lung adenocarcinoma via the miR-335–3p/CTHRC1 Axis. Cell Death Discov 8(1):98
Zhang R, Lu H, Lyu YY et al (2017) E6/E7-P53-POU2F1-CTHRC1 axis promotes cervical cancer metastasis and activates Wnt/PCP pathway. Sci Rep 7:44744
Baharom F, Ramirez-Valdez RA, Khalilnezhad A et al (2022) Systemic vaccination induces CD8(+) T cells and remodels the tumor microenvironment. Cell 185(23):4317–4332.e4315
Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, Zitvogel L (2016) Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol : Offic J Eur Soc Med Oncol 27(8):1482–1492
Acknowledgements
All cartoons were created with BioRender.com. We would like to thank Fei Chen, Chunjuan Bao, Qiqi Zhou, and Juan Mei (Institute of Clinical Pathology, West China Hospital of Sichuan University) for performing IHC and microscopic observation.
Funding
The National Natural Science Foundation of China (81602169). National Major Scientific and Technological Special Project (2018ZX09201018-021).
Author information
Authors and Affiliations
Contributions
YL conceived and designed the research. YW, XC, and YX performed experiments. TY and HW interpreted the results of the experiments. ZW analyzed the data. HZ prepared figures. LC drafted the paper. ZZ, JD, and RL edited and revised the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
The animal study was reviewed and approved by Animal Ethical and Welfare of West China Hospital, Sichuan University.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yixin Liu, Xiangzheng Chen, and Ying Xu have contributed equally to this work and share first authorship
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Chen, X., Xu, Y. et al. CTHRC1 promotes colorectal cancer progression by recruiting tumor-associated macrophages via up-regulation of CCL15. J Mol Med 102, 81–94 (2024). https://doi.org/10.1007/s00109-023-02399-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02399-0