Abstract
Macrophages belong to the innate immune system, and we have recently shown that in vitro differentiated human regulatory macrophages (Mreg) release large extracellular vesicles (L-EVMreg) with an average size of 7.5 μm which regulate wound healing and angiogenesis in vitro. The aim of this study was to investigate whether L-EVMreg also affect the CD3/CD28-mediated activation of T-cells. Mreg were differentiated using blood monocytes and L-EVMreg were isolated from culture supernatants by differential centrifugation. Activation of human T-cells was induced by CD3/CD28-coated beads in the absence or presence of Mreg or different concentrations of L-EVMreg. Inhibition of T-cell activation was quantified by flow cytometry and antibodies directed against the T-cell marker granzyme B. Phosphatidylserine (PS) exposure on the surface of Mreg and L-EVMreg was analyzed by fluorescence microscopy. Incubation of human lymphocytes with CD3/CD28 beads resulted in an increase of cell size, cell granularity, and number of granzyme B–positive cells (P < 0.05) which is indicative of T-cell activation. The presence of Mreg (0.5 × 106 Mreg/ml) led to a reduction of T-cell activation (number of granzyme B–positive cells; P < 0.001), and a similar but less pronounced effect was also observed when incubating activated T-cells with L-EVMreg (P < 0.05 for 3.2 × 106 L-EVMreg/ml). A differential analysis of the effects of Mreg and L-EVMreg on CD4+ and CD8+ T-cells showed an inhibition of CD4+ T-cells by Mreg (P < 0.01) and L-EVMreg (P < 0.05 for 1.6 × 106 L-EVMreg/ml; P < 0.01 for 3.2 × 106 L-EVMreg/ml). A moderate inhibition of CD8+ T-cells was observed by Mreg (P < 0.05) and by L-EVMreg (P < 0.01 for 1.6 × 106 L-EVMreg/ml and 3.2 × 106 L-EVMreg/ml). PS was restricted to confined regions of the Mreg surface, while L-EVMreg showed strong signals for PS in the exoplasmic leaflet. L-EVMreg attenuate CD3/CD28-mediated activation of CD4+ and CD8+ T-cells. L-EVMreg may have clinical relevance, particularly in the treatment of diseases associated with increased T-cell activity.
Key messages
-
Mreg release large extracellular vesicles (L-EVMreg) with an average size of 7.5 µm
-
L-EVMreg exhibit phosphatidylserine positivity
-
L-EVMreg suppress CD4+ and CD8+ T-cells
-
L-EVMreg hold clinical potential in T-cell-related diseases
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracellular vesicles (EV) are small structures ranging from nano- to micrometer in size that are released by almost all cell types [1, 2]. EV contain lipids, proteins, and RNAs, making them an efficient way to transfer functional cargoes and signals between cells [1,2,3,4]. Growing evidence suggests that EV play a critical role in complex communication among different cell types [2, 5]. Interestingly, large extracellular vesicles (L-EV), which range in diameter from 1 to 10 μm, have recently gained attention as potential sources of bioactive molecules and mediators of cell communication and angiogenesis [6, 7].
We have shown that human monocyte-derived regulatory macrophages (Mreg) contain and release pro-angiogenic proteins and produce large amounts of L-EVMreg as part of their differentiation process [8]. These L-EVMreg display an average size of 7.5 μm and an average volume of 0.2 pl. They carry distinct vesicular surface markers that characterize them as typical EV (LAMP-1, CD9, CD63, and CD81; based on MISEV guidelines 2018 [9]). In vitro, L-EVMreg are able to promote wound healing and have a positive effect on several parameters of angiogenesis which suggests that they could bear therapeutic potential for the treatment of chronic wounds and ischemia-associated diseases such as peripheral arterial occlusive disease [8].
It has been described that macrophages, among their numerous other functions, are also able to communicate with and regulate immune cells [10, 11]. In this context, Mreg can inhibit the activation of immune cells and have been successfully employed as therapeutic approach in recipients of kidney transplant to minimize the burden of general immunosuppression [12]. However, whether L-EVMreg are also able to regulate immune cells and which mechanisms are involved is still completely unclear. Here, we show that L-EVMreg are generated during the in vitro differentiation of monocytes to Mreg. L-EVMreg attenuate the CD3/CD28-induced activation of CD4+ and CD8+ T-cells and could therefore mediate immunomodulatory functions in vivo.
L-EVMreg can be easily isolated from Mreg cultures by differential centrifugation and could be therapeutically valuable for many different clinical indications and diseases that are associated with a dysregulated immune response (i.e., increased T-cell activity).
Materials and methods
Mreg differentiation and isolation of L-EVMreg
The study was approved by the local Ethics Committee of the University Medical Center Schleswig-Holstein, Kiel, Germany (protocol identification: D519/18 and D518/13). Peripheral blood mononuclear cells (PBMC) were obtained from leukocyte reduction system (LRS) chambers provided by the Department of Transfusion Medicine (University Hospital of Schleswig-Holstein, Kiel, Germany). Monocytes were isolated and differentiated to Mreg as described earlier [8] and depicted in Fig. 1A. Briefly, PBMC were purified through a Ficoll-Paque PLUS gradient (GE Healthcare, Chicago, USA) and monocytes were recovered using a CD14 magnetic bead cell sorting system (Miltenyi, Bergisch Gladbach, Germany) following the manufacturer’s protocol. The isolated CD14+ monocytes were cultivated in cell cultivation/differentiation bags (Miltenyi) at 0.83 × 106 cells/ml with RPMI 1640 medium containing GlutaMax (GIBCO, Billings, MT, USA) supplemented with 10% human AB-serum (Access Biological, Vista, CA, USA) and 2500 IU/ml human M-CSF (R&D Systems, Wiesbaden, Germany). After 6 days in culture, 500 IU/ml of human interferon (IFN) γ (R&D Systems, McKinley Place MN, USA) was added to the cultures and cells were incubated for additional 24 h. On day 7, Mreg were harvested and separated from culture medium by centrifugation (500xg for 10 min at room temperature; Fig. 1A). The pellets containing Mreg were resuspended in PBS and subjected to further analyses. The remaining culture supernatant containing EV was further centrifugated at 4000xg for 1 h at 4 °C and the L-EVMreg-containing pellet was resuspended in PBS and subjected to further analyses. Regarding Mreg and L-EVMreg recovery at day 7, typically around 16 × 106 Mreg and 31 × 106 L-EVMreg were obtained per 100-ml cultivation bag filled with 25ml culture medium.
Isolation and basic characterization of Mreg and L-EVMreg. A Schematic representation of the differentiation of Mreg from human monocytes and subsequent purification of L-EVMreg. B Results of the basic characterization of one representative Mreg and L-EVMreg fraction by flow cytometry and Coulter counter–based analysis. Mreg and LEVMreg can be distinguished as two distinct populations based on their granularity and size
Automated cell and vesicle analysis
Basic parameters such the number of particles (cells or vesicles) were evaluated using a MOXI cell counter (Orflo, Ketchum, ID, USA) which analyzes membrane surrounded structures within a size between 3 and 25 µm based on the Coulter principle (Fig. 1B, histogram).
In vitro T-cell activation assay
Employing the CD14+ monocyte isolation methodology detailed in this context, the selection column allowed the passage of exclusively CD14− cells. These cells were harvested and employed as a source for T-cell activation assays (Fig. 1A). For the activation process, CD14− cells were pre-incubated with CD3/CD28 activation beads (Dynabeads Human T activation, Gibco, MA, USA) for 15 min, following the manufacturer’s guidelines. Subsequently, CD14− cells (0.35 × 106/ml) were cultured either alone (activation control), in co-culture with Mreg (0.5 × 106/ml), or with varying concentrations of L-EVMreg (0.8, 1.6, and 3.2 × 106/ml). The decision for the used concentrations of L-EVMreg was based on the consideration that (i) the application of 0.5 × 106 Mreg/ml resulted in a statistically significant reduction of T-cell activation, and (ii) the L-EVMreg/Mreg ratio at the end of the differentiation period was 1.97 ± 0.64. After a co-incubation period of 3–5 days, supernatants containing non-adherent cells (including T-cells) were collected. The suspension was cleared of magnetic beads by positioning it near a magnetic field (MiniMACS™ separator, Miltenyi Biotec, Germany) for 5 min. Subsequently, the T-cells underwent flow cytometric analysis as described below (Fig. 2). For each T-cell activation assay, Mreg and L-EVMreg from the same preparation/batch were used. Mreg or L-EVMreg were not pooled.
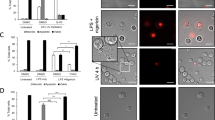
Schematic illustration of CD3/CD28-induced T-cell activation. A Administration of CD3/CD28 Dynabeads and subsequent binding to the corresponding surface receptors on resting T-cells lead to their activation. Using brightfield microscopy, activated T-cells dominate as clusters of large cells and are also detectable in the FSC/SSC flow cytometry blots. B Addition of Mreg or increasing concentrations of L-EVMreg to CD3/CD28-induced T-cells leads to inhibition of T-cell activation and lack of T-cell clustering
Flow cytometry
Cell or vesicle surface markers were analyzed in each L-EVMreg/Mreg preparation by flow cytometry before cells and vesicles were used for further experiments. Standard markers that were examined are CD31, CD206, CD11c, CD86, CD14, CD16, CD103, CD38, CD45, LAMP-1, CD9, CD63, and CD81. For details, refer to our previous publication [8].
Flow cytometry was performed using the MACS Q10™ cytometer (Miltenyi Biotec, Germany). Fluorescein isothiocyanate (FITC)–conjugated specific antibodies including anti-CD4 and anti-mouse IgG1κ (both from BD Biosciences), phycoerythrin (PE)-conjugated anti-granzyme B, anti-mouse IgG1κ (both from Invitrogen), and allophycocyanin (APC)-conjugated anti-CD8 and anti-mouse IgG1κ (both from BD Biosciences) were used. To assess viability, 7-AAD (BD Biosciences) staining was used.
Cells were incubated with anti-CD4 and anti-CD8 antibodies or their respective isotype controls for 30 min at 4 °C. For analyses of intracellular granzyme B, cells were fixed, permeabilized with eBioscience Fixation/Permeabilization Concentrate (Invitrogen, USA), and incubated with granzyme B or the isotype control antibodies for 20 min at 4 °C. The gating strategy consisted of selecting the respective singlet population on a FSC-A/FSC-H plot, choosing the T-cell population based on size and granularity (FSC/SSC profile), and evaluating the respective target protein taking into account the isotype control signal of 7-AAD-negative (living) cells.
To evaluate the exposure of PS on the outer cell membrane by flow cytometry, an annexin V-FITC kit (Miltenyi Biotec, Germany) was employed following the manufacturer’s instructions. Briefly, freshly isolated cells were incubated for 20 min with annexin V buffer and washed twice in the same buffer, and the final pellet was separated into four groups to incubate the cells (i) without any fluorochrome, (ii) with annexin V, (iii) with propidium iodide (PI), or (iv) with annexin V and PI. Annexin V was added and incubated for 20 min, while PI was added shortly before performing flow cytometry scans. Co-staining of cells with PI and annexin V was considered indicative of dead cells.
Fluorescence microscopy
For the visualization of PS in the outer cell/vesicle membrane, L-EVMreg-containing Mreg pellets were resuspended in a buffer consisting of 10 mM HEPES and 145 mM NaCl. The resuspended Mreg and L-EVMreg were supplemented with 10 µM of PSVue480 (Molecular Targeting Technologies, West Chester, PA, USA) and 0.2 ng/ml of Hoechst33342 for nuclei staining. The mixture was then kept in the dark at 37 °C for 15 min before being centrifuged at 500xg for 5 min. The resulting pellets were then resuspended in HEPES buffer. A small quantity of the resuspended Mreg and L-EVMreg was placed on a glass slide, covered with a glass coverslip, and immediately examined. The analysis was carried out using a Leica DM2000 LED fluorescent microscope equipped with DAPI, L5, and rhodamine filter cubes; a HC PL FLUOTAR × 40/0.80 objective; and a Leica DFC7000 T fluorescence camera, using the Image Overlay software.
Statistics
All experiments were carried out with cells and L-EVMreg derived from at least 5 healthy donors. The statistics software GraphPad Prism 5.01 for windows (GraphPad Software, San Diego, USA) was used to compare groups. All data were tested for normality using the Kolmogorov-Smirnov test. In cases normality was not obtained, the data were transformed (arcsin of square root of x) and analyzed using one-way ANOVA with Tukey test. A P-value < 0.05 was considered significant. All values are expressed as mean ± standard error mean (SEM).
Results
Basic characteristics of Mreg and L-EVMreg
The present study involves the characterization of monocyte-derived Mreg and L-EVMreg obtained after 7 days of differentiation. Mreg recovery rate at harvest and viability were 71.83 ± 5.91% and 83.54 ± 4.12%, respectively (data not shown). Visually, Mreg did not exhibit any signs of apoptosis such as blebbing or shrinkage and showed a typical morphology upon attachment to the culture well (Fig. 1A). Flow cytometry data revealed Mreg as highly viable granulated cell population, whereas L-EVMreg dominated as a defined, less granulated population lacking nuclei (Figs. 1B and 4). Using the Coulter principle–based MOXI cell counter, the presence of two distinct populations, one of 7.47 ± 0.75µm corresponding to L-EVMreg and the other of 13.73 ± 1.33µm corresponding to Mreg, could be confirmed (Fig. 1B). L-EVMreg are only present in Mreg cultures; the culture medium as well as the added human AB serum is devoid of L-EV (data not shown). A characterization of Mreg and L-EVMreg following the MISEV criteria [9] has been published by our group recently [8].
Effects of Mreg and L-EVMreg on CD3/CD28-induced T-cell activation
T-cells can be activated in vitro through the simultaneous binding of CD3 and CD28 to the corresponding receptors on the T-cell surface which is achieved by the addition of CD3/CD28 coated beads to the respective lymphocyte preparations [13]. Activated T-cells are easily identified in culture by an increase in cell size and granularity and the presence of cell accumulations. While clusters of activated T-cells are already visible using conventional brightfield microscopy, the increase in cell size and granularity can be evaluated by flow cytometry as a shift of the cell population toward higher FSC and SSC values. Using the described experimental setup (Fig. 2), we have analyzed the influence of Mreg and L-EVMreg on T-cell activation.
In a first step, T-cell activation was estimated based on an increase in cell size (FSC in flow cytometry) and cell granularity (SSC in flow cytometry). Addition of Mreg resulted in a significant reduction in the number of activated T-cells compared to the control (CD3/CD28 activated T-cell control, 100%; Mreg, 42.42 ± 5.99%; P < 0.0001; Fig. 3). The addition of L-EVMreg resulted in a dose-dependent inhibition of T-cell activation (CD3/CD28 activated T-cell control, 100%; 0.8 × 106 L-EVMreg/ml, 92.17 ± 3.29%, P > 0.05; 1.6 × 106 L-EVMreg/ml, 84.00 ± 3.53%, P < 0.05; 3.2 × 106 L-EVMreg/ml, 72.81 ± 4.80%, P < 0.05; Fig. 3).
Since activated T-cells express large amounts of intracellular granzyme B [14, 15], this enzyme was used as additional reliable intracellular marker to quantify the number of activated T-cells.
Our results show that the addition of Mreg causes a significant reduction in the number of activated T-cells compared to the control (CD3/CD28 activated T-cell control, 100%; Mreg, 17.29 ± 3.35%; P < 0.001; Fig. 4A). The addition of L-EVMreg resulted in a dose-dependent inhibition of T-cell activation, although a statistically significant effect could only be achieved with the highest concentration of L-EVMreg (CD3/CD28 activated T-cell control, 100%; 0.8 × 106 L-EVMreg/ml, 88.41 ± 9.38%, P > 0.05; 1.6 × 106 L-EVMreg/ml, 72.95 ± 17.51%, P > 0.05; 3.2 × 106 L-EVMreg/ml, 57.48 ± 13.30%, P < 0.05; Fig. 4A).
Effects of Mreg and L-EVMreg on the T-cell activation (intracellular granzyme B). A Relative number of activated T-cells. B Activated \({\mathrm{CD}4}^{+}\) T-cells. C Activated \({\mathrm{CD}8}^{+}\) T-cells. A representative flow cytometry dot plot is shown below the respective columns in B and C. *P < 0.05; **P < 0.01; ***P < 0.001 vs. activated T-cells (group: T-cells + CD3/CD28 beads)
Additional stratification of T-cells into CD4+ helper T-cells and CD8+ cytotoxic T-cells showed that both Mreg and L-EVMreg were able to inhibit CD3/CD28-induced activation of both T-cell subpopulations: CD4+ T-cells: CD3/CD28 activated CD4+ T-cell control, 58.42 ± 6.58% (granzyme B–positive cells); Mreg, 40.68 ± 4.19%, P < 0.01; 0.8 × 106 L-EVMreg/ml, 50.61 ± 7.72%, P > 0.05; 1.6 × 106 L-EVMreg/ml, 43.33 ± 11.18%, P < 0.05; 3.2 × 106 L-EVMreg/ml, 37.93 ± 6.78%, P < 0.01; Fig. 4B. CD8+ T-cells: CD3/CD28 activated CD8+ T-cell control, 26.89 ± 5.51% (granzyme B–positive cells); Mreg, 20.43 ± 3.48%, P < 0.05; 0.8 × 106 L-EVMreg/ml, 22.71 ± 4.77%, P > 0.05; 1.6 × 106 L-EVMreg/ml, 18.61 ± 5.15%, P < 0.01; 3.2 × 106 L-EVMreg/ml, 17.16 ± 3.37%, P < 0.01; Fig. 4C.
Another marker for the assessment of T-cell activation is CD25 which is upregulated on the surface of activated T-cells and serves as a high-affinity receptor for interleukin-2 (IL-2), a key cytokine involved in T-cell proliferation and survival [16]. In addition to the granzyme B evaluation, quantification of the number of CD25-positive T-cells also confirmed the above described effects of T-cell inhibition by Mreg and L-EVMreg (data not shown).
Phosphatidylserine exposure on the membrane of Mreg and L-EVMreg
As the exposure of phosphatidylserine (PS) by non-apoptotic cells has been recently recognized as an immunomodulatory mechanism [17] and PS is present in the cell membrane of non-apoptotic macrophages [18], we decided to analyze whether PS is also detectable in the outer lipid bilayer of Mreg and L-EVMreg. Nuclear staining for viable cells and PS staining were performed on Mreg and L-EVMreg. The results show that Mreg are either negative for PS or that PS is restricted to a confined region of the cell surface. In contrast to Mreg, L-EVMreg lack nuclei and show strong signals for PS on the entire surface (Fig. 5A–C). Extended analysis of the vesicular fraction by cytometry confirmed the fluorescence microscopy findings defining L-EVMreg as PS-positive vesicular structures devoid of nuclei.
Phosphatidylserine exposure on the membrane of Mreg and L-EVMreg. A Cells and vesicles were stained with Hoechst33342 (violet) and PSVue480 (green) to label DNA and PS, respectively. Signals were merged on the brightfield background to distinguish morphological features. Yellow arrows show L-EVMreg. Bar denotes 50 µm. B Left: magnified views of two selected areas from A. Right: same area as on the left showing only the nuclei staining. Yellow arrows denote L-EVMreg. Note that L-EVMreg lack nuclei and are positive for PS, while Mreg contain nuclei and are either negative for PS or reveal PS to be restricted to only a confined region of the cell surface. Bar denotes 20 µm. C Typical PS staining of a Mreg cell and one L-EVMreg. Yellow arrows denote L-EVMreg. Bar denotes 15 µm. D Left: Flow cytometry and gating (red box) of L-EVMreg. Right: Gated L-EVMreg are positive for PS (annexin V staining) and negative for nucleic acids (propidium iodide staining)
Discussion
Eukaryotic cells release extracellular vesicles (EV) into their microenvironment, which can vary in size and cargo composition [1, 2, 19]. EV transport a plethora of diverse biological molecules including lipids, carbohydrates, proteins, and RNAs, and their characteristics may vary depending on the conditions they are exposed to, allowing them to effectively transmit functional content and signals between cells [1, 2, 5, 20].
Macrophages are a type of white blood cell that play diverse roles in the immune response, including phagocytosis, antigen presentation, and regulation of inflammation. They can be polarized into different subsets, such as M1 and M2, which exhibit distinct functional and phenotypic characteristics [21, 22]. Macrophage-derived EV [23] have been found to play a role in numerous physiological and pathological pathways [24, 25] and to modulate immune responses [25,26,27].
Regulatory macrophages (Mreg), which in particular possess characteristics of anti-inflammatory M2 macrophages, can be generated from peripheral blood monocytes under defined growth factor-induced culture conditions [28,29,30]. Mreg secrete pro-angiogenic factors as well as various cytokines and have already been used in cell therapy–based trials to reduce organ rejection after kidney transplantation [12, 30]. In this context, intravenously applied Mreg minimized the burden of general immunosuppression after organ transplantation [12] and the underlying mechanisms may involve a Mreg-mediated inhibition of immune cell activation [10].
In our recent work, we have shown that monocyte-derived Mreg also secrete large EV (L-EVMreg), with an average diameter of 7.5 µm and an average volume of 0.22 pl, which contain pro-angiogenic molecules and induce angiogenesis and wound healing in vitro [8]. Here, we demonstrate for the first time that L-EVMreg are also able to attenuate CD3/CD28-induced activation of CD4+ and CD8+ T-cells.
T-cells play a crucial role in the immune system function by recognizing and responding to foreign antigens. Upon activation, they proliferate and differentiate into effector T-cells that coordinate an immune response against the pathogen [31]. Effector T-cells can be divided into helper and cytotoxic T-cells [32, 33]. Helper T-cells also known as CD4+ T-cells recognize and bind to antigen-presenting cells and provide help to other immune cells, such as B-cells and macrophages, to mount an effective immune response. They secrete cytokines that activate and coordinate the immune response against the pathogen [34, 35]. In contrast, cytotoxic T-cells, also known as CD8+ T-cells, recognize and kill infected or cancerous cells through the release of cytotoxic molecules. They play a crucial role in the elimination of intracellular pathogens and tumor cells [36]. Dysregulation of T-cell function can lead to various immune-related disorders, including autoimmune diseases and cancer [37,38,39].
Utilizing cytometric analyses, the current investigation revealed that both Mreg and L-EVMreg possess the ability to attenuate T-cell activation induced by CD3/CD28 stimulation. The suppressive potential of Mreg was found to be considerably greater compared to L-EVMreg; however, the latter still exhibited a statistically significant level of T-cell inhibition, particularly at the highest concentration of 3.2 × 106 L-EVMreg/ml employed. Despite the inferiority of L-EVMreg in terms of T-cell inhibition when compared to Mreg, L-EVMreg present numerous advantages for potential clinical applications: one major advantage of L-EVMreg is their lack of a cellular nucleus and inability to replicate. Consequently, L-EVMreg do not possess characteristics of living organisms, which reduces the likelihood of side effects that are commonly associated with the administration of autologous or allogeneic cells in a clinical context. Moreover, large quantities of L-EVMreg can be obtained from Mreg cultures as they are continually produced by Mreg and storage and handling of L-EVMreg are also considerably more convenient than those of vital Mreg cells. Finally, since L-EVMreg do not fall under the classification of Advanced Therapy Medicinal Products (ATMPs), they are exempt from the corresponding regulatory restrictions. This enables a faster and more convenient transition of a possible L-EVMreg treatment into clinical applications.
Regarding the eukaryotic lipid bilayer cell membrane, it is widely accepted that apoptosis triggers the exposure of PS on the outer membrane surface [40]. However, this distribution pattern of PS can also be observed in non-apoptotic cells [41] and it was shown that PS expressed on the surface of human exosomes is linked to T-cell immunosuppression [40, 42]. Therefore, it could be proposed that the exposure of PS in the exoplasmic leaflet of Mreg and L-EVMreg described in the present work may be involved in the Mreg- and L-EVMreg-mediated attenuation of T-cell activation. Also, the PD-1/PD-L1 system, which plays an important role in regulating several components of the immune system [43,44,45], might be involved in T-cell regulation. Preliminary findings of our group showed that 82.6 ± 3.8% of Mreg and 22.0 ± 6.1% of L-EVMreg are positive for PD-L1, the ligand for PD-1 which is expressed on the surface of T-cells [43, 46]. Although the above-mentioned potential mechanisms of T-cell inhibition by L-EVMreg are scientifically of interest, they are of only secondary importance for the potential clinical application of L-EVMreg and were not the main objective of our present work.
It should be noted that despite intensive characterization of L-EVMreg, their categorization into the different classes of EV is not fully established at this stage. However, there are several similarities between exophers and L-EVMreg [47]. Exophers are several micrometers in size and have been demonstrated to detach from cells within a matter of hours [48]. Unfortunately, we are unable to investigate this characteristic trait of exophers in the context of L-EVMreg. Throughout the differentiation phase, Mreg remain within the culture bags inside the incubator, rendering them inaccessible to morphological analyses. Furthermore, even the simple act of transferring the bags from the incubator to the microscope is likely to dissolve any potential connection between the exophers and their parent cells. However, exophers typically possess a lipid bilayer and exhibit phosphatidylserine on their surface, thereby sharing certain characteristics with the L-EVMreg described in our study [48].
In conclusion, the present study has revealed the novel finding that PS positive L-EVMreg can suppress both CD4+ and CD8+ T-cells, suggesting the potential clinical use of L-EVMreg in various diseases associated with increased T-cell activity, including multiple sclerosis, rheumatoid arthritis, type 1 diabetes, allergic reactions, and transplant rejection.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Teng F, Fussenegger M (2020) Shedding light on extracellular vesicle biogenesis and bioengineering. Adv Sci (Weinh) 8:2003505. https://doi.org/10.1002/advs.202003505
Kang T, Atukorala I, Mathivanan S (2021) Biogenesis of extracellular vesicles. Subcell Biochem 97:19–43. https://doi.org/10.1007/978-3-030-67171-6_2
Chang X, Fang L, Bai J, Wang Z (2020) Characteristics and changes of DNA in extracellular vesicles. DNA Cell Biol 39:1486–1493. https://doi.org/10.1089/dna.2019.5005
Heydari Z, Peshkova M, Gonen ZB, Coretchi I, Eken A, Yay AH, Dogan ME, Gokce N, Akalin H, Kosheleva N et al (2023) EVs vs. EVs: MSCs and Tregs as a source of invisible possibilities. J Mol Med (Berl) 101:51–63. https://doi.org/10.1007/s00109-022-02276-2
Weng Q, Wang Y, Xie Y, Yu X, Zhang S, Ge J, Li Z, Ye G, Guo J (2022) Extracellular vesicles-associated tRNA-derived fragments (tRFs): biogenesis, biological functions, and their role as potential biomarkers in human diseases. J Mol Med (Berl) 100:679–695. https://doi.org/10.1007/s00109-022-02189-0
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F (2017) Extracellular vesicles in angiogenesis. Circ Res 120:1658–1673. https://doi.org/10.1161/CIRCRESAHA.117.309681
Slomka A, Urban SK, Lukacs-Kornek V, Zekanowska E, Kornek M (2018) Large extracellular vesicles: have we found the holy grail of inflammation? Front Immunol 9:2723. https://doi.org/10.3389/fimmu.2018.02723
Albrecht M, Hummitzsch L, Rusch R, Hess K, Steinfath M, Cremer J, Lichte F, Fandrich F, Berndt R, Zitta K (2023) Characterization of large extracellular vesicles (L-EV) derived from human regulatory macrophages (Mreg): novel mediators in wound healing and angiogenesis? J Transl Med 21:61. https://doi.org/10.1186/s12967-023-03900-6
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7:1535750. https://doi.org/10.1080/20013078.2018.1535750
Riquelme P, Govert F, Geissler EK, Fandrich F, Hutchinson JA (2009) Human transplant acceptance-inducing cells suppress mitogen-stimulated T cell proliferation. Transpl Immunol 21:162–165. https://doi.org/10.1016/j.trim.2009.03.004
Salminen A (2021) Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med (Berl) 99:1553–1569. https://doi.org/10.1007/s00109-021-02123-w
Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, Tang Q, Guinan EC, Battaglia M, Burlingham WJ et al (2020) Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 395:1627–1639. https://doi.org/10.1016/S0140-6736(20)30167-7
Tumaini B, Lee DW, Lin T, Castiello L, Stroncek DF, Mackall C, Wayne A, Sabatino M (2013) Simplified process for the production of anti-CD19-CAR-engineered T cells. Cytotherapy 15:1406–1415. https://doi.org/10.1016/j.jcyt.2013.06.003
Mellor-Heineke S, Villanueva J, Jordan MB, Marsh R, Zhang K, Bleesing JJ, Filipovich AH, Risma KA (2013) Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front Immunol 4:72. https://doi.org/10.3389/fimmu.2013.00072
Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ (2004) Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104:2840–2848. https://doi.org/10.1182/blood-2004-03-0859
Cantrell D (2015) Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol 7. https://doi.org/10.1101/cshperspect.a018788
Shlomovitz I, Speir M, Gerlic M (2019) Flipping the dogma - phosphatidylserine in non-apoptotic cell death. Cell Commun Signal 17:139. https://doi.org/10.1186/s12964-019-0437-0
Callahan MK, Williamson P, Schlegel RA (2000) Surface expression of phosphatidylserine on macrophages is required for phagocytosis of apoptotic thymocytes. Cell Death Differ 7:645–653. https://doi.org/10.1038/sj.cdd.4400690
Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol 36:301–312. https://doi.org/10.1007/s10571-016-0366-z
Akers JC, Gonda D, Kim R, Carter BS, Chen CC (2013) Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 113:1–11. https://doi.org/10.1007/s11060-013-1084-8
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737. https://doi.org/10.1038/nri3073
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. https://doi.org/10.1038/nri2448
Dechantsreiter S, Ambrose AR, Worboys JD, Lim JME, Liu S, Shah R, Montero MA, Quinn AM, Hussell T, Tannahill GM et al (2022) Heterogeneity in extracellular vesicle secretion by single human macrophages revealed by super-resolution microscopy. J Extracell Vesicles 11:e12215. https://doi.org/10.1002/jev2.12215
Wang Y, Zhao M, Liu S, Guo J, Lu Y, Cheng J, Liu J (2020) Macrophage-derived extracellular vesicles: diverse mediators of pathology and therapeutics in multiple diseases. Cell Death Dis 11:924. https://doi.org/10.1038/s41419-020-03127-z
Xing Y, Sun X, Dou Y, Wang M, Zhao Y, Yang Q, Zhao Y (2021) The immuno-modulation effect of macrophage-derived extracellular vesicles in chronic inflammatory diseases. Front Immunol 12:785728. https://doi.org/10.3389/fimmu.2021.785728
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, Krasnodembskaya AD (2017) Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med 196:1275–1286. https://doi.org/10.1164/rccm.201701-0170OC
Tang D, Cao F, Yan C, Fang K, Ma J, Gao L, Sun B, Wang G (2022) Extracellular vesicle/macrophage axis: potential targets for inflammatory disease intervention. Front Immunol 13:705472. https://doi.org/10.3389/fimmu.2022.705472
Gurvich OL, Puttonen KA, Bailey A, Kailaanmaki A, Skirdenko V, Sivonen M, Pietikainen S, Parker NR, Yla-Herttuala S, Kekarainen T (2020) Transcriptomics uncovers substantial variability associated with alterations in manufacturing processes of macrophage cell therapy products. Sci Rep 10:14049. https://doi.org/10.1038/s41598-020-70967-2
Hutchinson JA, Ahrens N, Geissler EK (2017) MITAP-compliant characterization of human regulatory macrophages. Transpl Int 30:765–775. https://doi.org/10.1111/tri.12988
Hummitzsch L, Zitta K, Rusch R, Cremer J, Steinfath M, Gross J, Fandrich F, Berndt R, Albrecht M (2019) Characterization of the angiogenic potential of human regulatory macrophages (Mreg) after ischemia/reperfusion injury in vitro. Stem Cells Int 2019:3725863. https://doi.org/10.1155/2019/3725863
Krogsgaard M, Davis MM (2005) How T cells ‘see’ antigen. Nat Immunol 6:239–245. https://doi.org/10.1038/ni1173
Harty JT, Badovinac VP (2008) Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol 8:107–119. https://doi.org/10.1038/nri2251
Williams MA, Bevan MJ (2007) Effector and memory CTL differentiation. Annu Rev Immunol 25:171–192. https://doi.org/10.1146/annurev.immunol.25.022106.141548
Wan YY, Flavell RA (2007) ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev 220:199–213. https://doi.org/10.1111/j.1600-065X.2007.00565.x
Vignali DA, Collison LW, Workman CJ (2008) How regulatory T cells work. Nat Rev Immunol 8:523–532. https://doi.org/10.1038/nri2343
Halle S, Halle O, Forster R (2017) Mechanisms and dynamics of T cell-mediated cytotoxicity in vivo. Trends Immunol 38:432–443. https://doi.org/10.1016/j.it.2017.04.002
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747. https://doi.org/10.1146/annurev.immunol.23.021704.115707
Atkinson MA, Eisenbarth GS, Michels AW (2014) Type 1 diabetes. Lancet 383:69–82. https://doi.org/10.1016/S0140-6736(13)60591-7
Wherry EJ, Kurachi M (2015) Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15:486–499. https://doi.org/10.1038/nri3862
Bhatta M, Shenoy GN, Loyall JL, Gray BD, Bapardekar M, Conway A, Minderman H, Kelleher RJ, Jr., Carreno BM, Linette G et al (2021) Novel phosphatidylserine-binding molecule enhances antitumor T-cell responses by targeting immunosuppressive exosomes in human tumor microenvironments. J Immunother Cancer 9. https://doi.org/10.1136/jitc-2021-003148
Thery C, Ostrowski M, Segura E (2009) Membrane vesicles as conveyors of immune responses. Nat Rev Immunol 9:581–593. https://doi.org/10.1038/nri2567
Zhao X, Yuan C, Wangmo D, Subramanian S (2021) Tumor-secreted extracellular vesicles regulate T-cell costimulation and can be manipulated to induce tumor-specific T-cell responses. Gastroenterology 161:560–574 e511. https://doi.org/10.1053/j.gastro.2021.04.036
Sharpe AH, Pauken KE (2018) The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol 18:153–167. https://doi.org/10.1038/nri.2017.108
Roux C, Jafari SM, Shinde R, Duncan G, Cescon DW, Silvester J, Chu MF, Hodgson K, Berger T, Wakeham A et al (2019) Reactive oxygen species modulate macrophage immunosuppressive phenotype through the up-regulation of PD-L1. Proc Natl Acad Sci U S A 116:4326–4335. https://doi.org/10.1073/pnas.1819473116
Ai L, Chen J, Yan H, He Q, Luo P, Xu Z, Yang X (2020) Research status and outlook of PD-1/PD-L1 inhibitors for cancer therapy. Drug Des Devel Ther 14:3625–3649. https://doi.org/10.2147/DDDT.S267433
Konkel JE, Frommer F, Leech MD, Yagita H, Waisman A, Anderton SM (2010) PD-1 signalling in CD4(+) T cells restrains their clonal expansion to an immunogenic stimulus, but is not critically required for peptide-induced tolerance. Immunology 130:92–102. https://doi.org/10.1111/j.1365-2567.2009.03216.x
Arnold ML, Cooper J, Grant BD, Driscoll M (2020) Quantitative approaches for scoring in vivo neuronal aggregate and organelle extrusion in large exopher vesicles in C. elegans. J Vis Exp. https://doi.org/10.3791/61368
Jeppesen DK, Zhang Q, Franklin JL, Coffey RJ (2023) Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol 33:667–681. https://doi.org/10.1016/j.tcb.2023.01.002
Acknowledgements
We thank Kerstin Parczany, Kerstin Marx, and Christopher Schnoor for excellent technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by a grant from Trizell GmbH, Hamburg, Germany.
Author information
Authors and Affiliations
Contributions
Study concept and design: MA, RB, and KZ. Practical implementation of experiments: LH, RB, KH, and KZ. Data analyses and statistical analyses: LH, RR, KH, RB, and MA. Writing of the manuscript: KZ and MA. Critical revision of the manuscript: MS, JC, MR, CE, and FF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the local Ethics Committee of the University Medical Center Schleswig-Holstein, Kiel, Germany (protocol identification: D519/18 and D518/13).
Consent for publication
Not applicable.
Competing interests
The authors (MA, KZ, FF, RB) are involved in a pending patent (European Patent Office, Nr. 23153832.3 Mreg-derived vesicles). All other authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albrecht, M., Hummitzsch, L., Rusch, R. et al. Large extracellular vesicles derived from human regulatory macrophages (L-EVMreg) attenuate CD3/CD28-induced T-cell activation in vitro. J Mol Med 101, 1437–1448 (2023). https://doi.org/10.1007/s00109-023-02374-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02374-9