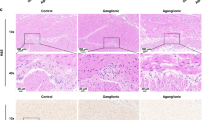
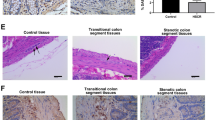
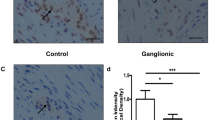
Abstract
Hirschsprung disease (HSCR) is a congenital disorder caused by the failure of enteric neural crest cells (ENCCs) to colonize the distal bowel, resulting in absence of enteric nervous system. While a range of molecules and signaling pathways have been found to contribute to HSCR development, the risk factors and pathogenesis of this disease in many patients remain unknown. We previously demonstrated that increased activity of the prostaglandin E2 (PGE2)/PGE2 receptor subtype EP2 pathway can be a risk factor for HSCR. In this study, an Ednrb-deficient mouse model of HSCR was generated and used to investigate if PGE2/EP2 pathway could be a potential therapeutic target for HSCR. We found that downregulation of PGE2/EP2 signaling by siRNA-mediated ablation of a PGE2 synthase or pharmacologic blockage of EP2 enhanced ENCC colonization in the distal bowel of Ednrb−/− mice and alleviated their HSCR-like symptoms. Furthermore, blockage of EP2 was shown to promote ENCC migration through upregulating p38 mitogen-activated protein kinase activity, which was downregulated in the colon of Ednrb−/− mice and in the distal aganglionic bowel of HSCR patients. These data provide evidence that maternal exposure during embryonic development to an environment with dysregulated activation of the PGE2/EP2 pathway may predispose genetically susceptible offspring to HSCR, and avoidance or early disruption of maternal events (e.g. inflammation) that possibly enhance PGE2/EP2 signaling during pregnancy would reduce the occurrence and severity of this disease.
Key messages
-
Knockdown of PTGES alleviates HSCR severity in Ednrb−/− mice.
-
Blockage of EP2-mediated PGE2 signaling alleviates HSCR severity in Ednrb−/− mice.
-
Blockage of EP2-mediated PGE2 signaling promotes ENCC migration via enhancing p38 activity.






Similar content being viewed by others
Data availability
All data for this work are included within the article and its supplementary materials. Raw data are available upon request to the corresponding authors.
Code availability
Not applicable.
References
Heuckeroth RO (2018) Hirschsprung disease - integrating basic science and clinical medicine to improve outcomes. Nat Rev Gastroenterol Hepatol 15:152–167
Butler Tjaden NE, Trainor PA (2013) The developmental etiology and pathogenesis of Hirschsprung disease. Transl Res 162:1–15
Luzón-Toro B, Villalba-Benito L, Torroglosa A, Fernández RM, Antiñolo G, Borrego S (2020) What is new about the genetic background of Hirschsprung disease? Clin Genet 97:114–124
Bondurand N, Southard-Smith EM (2016) Mouse models of Hirschsprung disease and other developmental disorders of the enteric nervous system: Old and new players. Dev Biol 417:139–157
Alves MM, Sribudiani Y, Brouwer RW, Amiel J, Antiñolo G, Borrego S, Ceccherini I, Chakravarti A, Fernández RM, Garcia-Barcelo MM et al (2013) Contribution of rare and common variants determine complex diseases-Hirschsprung disease as a model. Dev Biol 382:320–329
Heuckeroth RO, Schäfer KH (2016) Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention. Dev Biol 417:188–197
Lake JI, Tusheva OA, Graham BL, Heuckeroth RO (2013) Hirschsprung-like disease is exacerbated by reduced de novo GMP synthesis. J Clin Invest 123:4875–4887
Tang W, Chen M, Guo X, Zhou K, Wen Z, Liu F, Liu X, Mao X, He X, Hu W et al (2021) Multiple ’omics’-analysis reveals the role of prostaglandin E2 in Hirschsprung’s disease. Free Radic Biol Med 164:390–398
Bergeron KF, Cardinal T, Pilon N (2013) A quantitative cell migration assay for murine enteric neural progenitors. J Vis Exp 18:50709
Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M (1994) Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell 79:1267–1276
Chowdhury MK, Wu LE, Coleman JL, Smith NJ, Morris MJ, Shepherd PR, Smith GC (2016) Niclosamide blocks glucagon phosphorylation of Ser552 on β-catenin in primary rat hepatocytes via PKA signalling. Biochem J 473:1247–1255
Af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, Pullen N (2011) In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP2 receptor antagonist. Br J Pharmacol 164:1847–1856
Lake JI, Heuckeroth RO (2013) Enteric nervous system development: migration, differentiation, and disease. Am J Physiol Gastrointest Liver Physiol 305:2
Bondurand N, Dufour S, Pingault V (2018) News from the endothelin-3/EDNRB signaling pathway: Role during enteric nervous system development and involvement in neural crest-associated disorders. Dev Biol 1:S156–S169
Yousif NM, de Oliveira ACP, Brioschi S, Huell M, Biber K, Fiebich BL (2018) Activation of EP(2) receptor suppresses poly(I: C) and LPS-mediated inflammation in primary microglia and organotypic hippocampal slice cultures: Contributing role for MAPKs. Glia 66:708–724
Kawashita E, Tsuji D, Toyoshima M, Kanno Y, Matsuno H, Itoh K (2011) Prostaglandin E2 reverses aberrant production of an inflammatory chemokine by microglia from Sandhoff disease model mice through the cAMP-PKA pathway. PLoS ONE 6:0016269
Kawahara K, Hohjoh H, Inazumi T, Tsuchiya S, Sugimoto Y (2015) Prostaglandin E2-induced inflammation: Relevance of prostaglandin E receptors. Biochim Biophys Acta 4:414–421
Keene CD, Chang R, Stephen C, Nivison M, Nutt SE, Look A, Breyer RM, Horner PJ, Hevner R, Montine TJ (2009) Protection of hippocampal neurogenesis from toll-like receptor 4-dependent innate immune activation by ablation of prostaglandin E2 receptor subtype EP1 or EP2. Am J Pathol 174:2300–2309
Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, Brown HD, Mhatre SD, Loui T, Andreasson KI (2015) Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. J Clin Invest 125:350–364
Johansson JU, Pradhan S, Lokteva LA, Woodling NS, Ko N, Brown HD, Wang Q, Loh C, Cekanaviciute E et al (2013) Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci 33:16016–16032
Liu Q, Liang X, Wang Q, Wilson EN, Lam R, Wang J, Kong W, Tsai C, Pan T, Larkin PB et al (2019) PGE(2) signaling via the neuronal EP2 receptor increases injury in a model of cerebral ischemia. Proc Natl Acad Sci USA 116:10019–10024
Li L, Yu Y, Hou R, Hao J, Jiang J (2020) Inhibiting the PGE(2) Receptor EP2 Mitigates Excitotoxicity and Ischemic Injury. ACS Pharmacol Transl Sci 3:635–643
Schill EM, Lake JI, Tusheva OA, Nagy N, Bery SK, Foster L, Avetisyan M, Johnson SL, Stenson WF et al (2016) Ibuprofen slows migration and inhibits bowel colonization by enteric nervous system precursors in zebrafish, chick and mouse. Dev Biol 409:473–488
Chatterjee S, Chakravarti A (2019) A gene regulatory network explains RET-EDNRB epistasis in Hirschsprung disease. Hum Mol Genet 28:3137–3147
Segarra J, Balenci L, Drenth T, Maina F, Lamballe F (2006) Combined signaling through ERK, PI3K/AKT, and RAC1/p38 is required for met-triggered cortical neuron migration. J Biol Chem 281:4771–4778
Allen MP, Linseman DA, Udo H, Xu M, Schaack JB, Varnum B, Kandel ER, Heidenreich KA, Wierman ME (2002) Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase. Mol Cell Biol 22:599–613
Hamanoue M, Morioka K, Ohsawa I, Ohsawa K, Kobayashi M, Tsuburaya K, Akasaka Y, Mikami T, Ogata T, Takamatsu K (2016) Cell-permeable p38 MAP kinase promotes migration of adult neural stem/progenitor cells. Sci Rep 6
Sato A, Scholl AM, Kuhn EN, Stadt HA, Decker JR, Pegram K, Hutson MR, Kirby ML (2011) FGF8 signaling is chemotactic for cardiac neural crest cells. Dev Biol 354:18–30
Funding
This work was supported by Southeast University-Nanjing Medical University Collaborative Research Fund (No. 2242017K3DN08 to XM and WT), the National Natural Science Foundation of China (No. 81570467 to WT), and the Open Project of the Key Laboratory of Developmental Genes and Human Diseases, Ministry of Education, China (No. LDGHD202203 to JC).
Author information
Authors and Affiliations
Contributions
JW, ZZ, NJ, YH, JC and HL conducted the experiments and were responsible for data acquisition. JD provided technical support. JT provided human samples. JW, NJ, ZZ, JD, YH, WT and XM were involved in analysis and interpretation of data. XM wrote the manuscript. ZZ contributed to the writing of Materials and Methods. JW and YH prepared the figures. XM and WT conceived and supervised the study. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
All animal experiments were performed following the guideline of the Animal Care & Welfare Committee of Southeast University, China, and the Institutional Animal Care and Use Committee of Nanjing Medical University, China. Studies involving human subjects were approved by the Ethics Committee of Nanjing Medical University (Approval Number: 2015-90).
Consent to participate
Written informed consent was obtained from children’s parents.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 1352 KB)
Supplementary file3 (MP4 780 KB)
Supplementary file4 (MP4 2953 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Zhi, Z., Ding, J. et al. Suppression of PGE2/EP2 signaling alleviates Hirschsprung disease by upregulating p38 mitogen-activated protein kinase activity. J Mol Med 101, 1125–1139 (2023). https://doi.org/10.1007/s00109-023-02353-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02353-0