Abstract
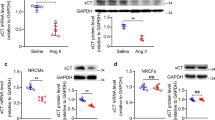
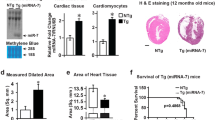
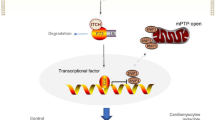
TRIM-containing 44 (TRIM44) is a promoter of multiple cancers. However, its role in cardiac hypertrophy has not been elucidated. This study explored the role of TRIM44 on pressure overload-induced cardiac hypertrophy in mice. Mice were subjected to aortic banding to establish an adverse cardiac hypertrophy model, followed by the administration of AAV9-TRIM44 or AAV9shTRIM44 to overexpress or knock down TRIM44. Echocardiography was used to assess cardiac function. H9c2 cells were cultured and transfected with either Ad-TRIM44 or TRIM44 siRNA to overexpress or silence TRIM44. Cells were also stimulated with angiotensin II to establish a cardiomyocyte hypertrophy model. Results indicated that TRIM44 was downregulated in mice hearts and cardiomyocytes that were treated with aortic banding or angiotensin II. TRIM44 overexpression in mice hearts aggravated cardiac hypertrophy and fibrosis, as well as inhibited cardiac function post-aortic banding. Moreover, mice with TRIM44 overexpression displayed increased ferroptosis post-aortic banding. Mice with TRIM44 knockdown revealed ameliorated cardiac hypertrophy, ferroptosis, and fibrosis, as well as improved cardiac function post-aortic banding. In H9c2 cells transfected with Ad-TRIM44, angiotensin II-induced ferroptosis was enhanced, while cells with silenced TRIM44 reported reduced ferroptosis post-angiotensin II administration. Furthermore, TRIM44 interacted with TLR4, which increased the expression of NOX4 and subsequently augmented ferroptosis-associated protein levels. By using TLR4 knockout mice, the inhibitory role of TRIM44 was reduced post-aortic banding. Taken together, TRIM44 aggravated pressure overload-induced cardiac hypertrophy via increased TLR4/NOX4-associated ferroptosis.
Key messages
-
TRIM44 could aggregate pressure overload-induced cardiac hypertrophy via increasing TLR4-NOX4 associated ferroptosis.
-
Target TRIM44 may become a new therapeutic method for preventing or treating pressure overload-induced cardiac hypertrophy.









Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Yang D, Liu HQ, Liu FY, Guo Z, An P, Wang MY, Yang Z, Fan D, Tang QZ (2021) Mitochondria in Pathological cardiac hypertrophy research and therapy. Front Cardiovasc Med 8:822969
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407
Zhu L, Li C, Liu Q, Xu W, Zhou X (2019) Molecular biomarkers in cardiac hypertrophy. J Cell Mol Med 23:1671–1677
Lyon RC, Zanella F, Omens JH, Sheikh F (2015) Mechanotransduction in cardiac hypertrophy and failure. Circ Res 116:1462–1476
Matsuura TR, Leone TC, Kelly DP (2020) Fueling cardiac hypertrophy. Circ Res 126:197–199
Liu J, Li W, Deng KQ, Tian S, Liu H, Shi H, Fang Q, Liu Z, Chen Z, Tian T et al (2022) The E3 ligase TRIM16 is a key suppressor of pathological cardiac hypertrophy. Circ Res 130:1586–1600
Sparrer KMJ, Gableske S, Zurenski MA, Parker ZM, Full F, Baumgart GJ, Kato J, Pacheco-Rodriguez G, Liang C, Pornillos O et al (2017) TRIM23 mediates virus-induced autophagy via activation of TBK1. Nat Microbiol 2:1543–1557
Li Y, Meng Q, Wang L, Cui Y (2021) TRIM27 protects against cardiac ischemia-reperfusion injury by suppression of apoptosis and inflammation via negatively regulating p53. Biochem Biophys Res Commun 557:127–134
Lyu L, Chen Z, McCarty N (2022) TRIM44 links the UPS to SQSTM1/p62-dependent aggrephagy and removing misfolded proteins. Autophagy 18:783–798
Yu XZ, Yuan JL, Ye H, Yi K, Qie MR, Hou MM (2021) TRIM44 facilitates ovarian cancer proliferation, migration, and invasion by inhibiting FRK. Neoplasma 68:751–759
Luo F, Wu Y, Zhu L, Zhang J, Liu Y, Jia W (2020) Knockdown of HIF1A-AS2 suppresses TRIM44 to protect cardiomyocytes against hypoxia-induced injury. Cell Biol Int 44:1523–1534
Gao L, Liu Y, Guo S, Xiao L, Wu L, Wang Z, Liang C, Yao R, Zhang Y (2018) LAZ3 protects cardiac remodeling in diabetic cardiomyopathy via regulating miR-21/PPARa signaling. Biochim Biophys Acta Mol Basis Dis 1864:3322–3338
Jiang XY, Guan FF, Ma JX, Dong W, Qi XL, Zhang X, Chen W, Gao S, Gao X, Pan S et al (2023) Cardiac-specific Trim44 knockout in rat attenuates isoproterenol-induced cardiac remodeling via inhibition of AKT/mTOR pathway. Dis Model Mech 16
Wei CY, Wang L, Zhu MX, Deng XY, Wang DH, Zhang SM, Ying JH, Yuan X, Wang Q, Xuan TF et al (2019) TRIM44 activates the AKT/mTOR signal pathway to induce melanoma progression by stabilizing TLR4. J Exp Clin Cancer Res 38:137
Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D (2016) Ferroptosis: process and function. Cell Death Differ 23:369–379
Li N, Jiang W, Wang W, Xiong R, Wu X, Geng Q (2021) Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol Res 166:105466
Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X et al (2019) Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci U S A 116:2672–2680
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C, Wu H, Deng W, Shen D, Tang Q (2020) Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic Biol Med 160:303–318
Wang J, Deng B, Liu Q, Huang Y, Chen W, Li J, Zhou Z, Zhang L, Liang B, He J et al (2020) Pyroptosis and ferroptosis induced by mixed lineage kinase 3 (MLK3) signaling in cardiomyocytes are essential for myocardial fibrosis in response to pressure overload. Cell Death Dis 11:574
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Cadenas S (2018) ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic Biol Med 117:76–89
Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS (2021) NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol 41:101947
Chen X, Xu S, Zhao CLiu B (2019) Role of TLR4/NADPH oxidase 4 pathway in promoting cell death through autophagy and ferroptosis during heart failure. Biochem Biophys Res Commun 516:37–43
de Vicente LG, Munoz VR, Pinto AP, Rovina RL, da Rocha AL, Marafon BB, Tavares MEA, Teixeira GR, Ferrari GD, Alberici LC et al (2021) TLR4 deletion increases basal energy expenditure and attenuates heart apoptosis and ER stress but mitigates the training-induced cardiac function and performance improvement. Life Sci 285:119988
Suzuki Y, Hattori K, Hamanaka J, Murase T, Egashira Y, Mishiro K, Ishiguro M, Tsuruma K, Hirose Y, Tanaka H et al (2012) Pharmacological inhibition of TLR4-NOX4 signal protects against neuronal death in transient focal ischemia. Sci Rep 2:896
Funding
This research was supported by the Henan Science and Technology Tackling Plan—Joint Construction Project [No. LHGJ20190100].
Author information
Authors and Affiliations
Contributions
Lu Gao and Yanzhou Zhang contributed to the conception and design of the experiments; Lu Gao, Leiming Wu, Lili Xiao, and Rui Yao carried out the experiments; Lili Xiao, Leiming Wu, and Zheng Wang analyzed the experimental results. Rui Yao wrote and revised the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
The animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and were approved by the Animal Care and Use Committee of the First Affiliated Hospital of Zhengzhou University (ZH-2021–0120).
Consent to participate
Not applicable.
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, L., Jia, M., Xiao, L. et al. TRIM-containing 44 aggravates cardiac hypertrophy via TLR4/NOX4-induced ferroptosis. J Mol Med 101, 685–697 (2023). https://doi.org/10.1007/s00109-023-02318-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02318-3