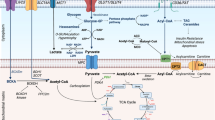
Abstract
Ischemia-induced metabolic remodeling plays a critical role in the pathogenesis of adverse cardiac remodeling and heart failure however, the underlying molecular mechanism is largely unknown. Here, we assess the potential roles of nicotinamide riboside kinase-2 (NRK-2), a muscle-specific protein, in ischemia-induced metabolic switch and heart failure through employing transcriptomic and metabolomic approaches in ischemic NRK-2 knockout mice. The investigations revealed NRK-2 as a novel regulator of several metabolic processes in the ischemic heart. Cardiac metabolism and mitochondrial function and fibrosis were identified as top dysregulated cellular processes in the KO hearts post-MI. Several genes linked to mitochondrial function, metabolism, and cardiomyocyte structural proteins were severely downregulated in the ischemic NRK-2 KO hearts. Analysis revealed significantly upregulated ECM-related pathways which was accompanied by the upregulation of several key cell signaling pathways including SMAD, MAPK, cGMP, integrin, and Akt in the KO heart post-MI. Metabolomic studies identified profound upregulation of metabolites mevalonic acid, 3,4-dihydroxyphenylglycol, 2-penylbutyric acid, and uridine. However, other metabolites stearic acid, 8,11,14-eicosatrienoic acid, and 2-pyrrolidinone were significantly downregulated in the ischemic KO hearts. Taken together, these findings suggest that NRK-2 promotes metabolic adaptation in the ischemic heart. The aberrant metabolism in the ischemic NRK-2 KO heart is largely driven by dysregulated cGMP and Akt and mitochondrial pathways.
Key messages
-
Post-myocardial infarction metabolic switch critically regulates the pathogenesis of adverse cardiac remodeling and heart failure. Here, we report NRK-2 as a novel regulator of several cellular processes including metabolism and mitochondrial function post-MI. NRK-2 deficiency leads to downregulation of genes important for mitochondrial pathway, metabolism, and cardiomyocyte structural proteins in the ischemic heart. It was accompanied by upregulation of several key cell signaling pathways including SMAD, MAPK, cGMP, integrin, and Akt and dysregulation of numerous metabolites essential for cardiac bioenergetics. Taken together, these findings suggest that NRK-2 is critical for metabolic adaptation of the ischemic heart.






Similar content being viewed by others
Data availability
Data is included in the manuscript and supplementary files. All the raw data is deposited in the NCBI database with the accession number GSE223868.
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141:e139–e596. https://doi.org/10.1161/CIR.0000000000000757
Tran DH, Wang ZV (2019) Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc 8:e012673. https://doi.org/10.1161/JAHA.119.012673
Lal H, Ahmad F, Woodgett J, Force T (2015) The GSK-3 family as therapeutic target for myocardial diseases. Circ Res 116:138–149. https://doi.org/10.1161/CIRCRESAHA.116.303613
Lee L, Horowitz J, Frenneaux M (2004) Metabolic manipulation in ischaemic heart disease, a novel approach to treatment. Eur Heart J 25:634–641. https://doi.org/10.1016/j.ehj.2004.02.018
Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD (1995) High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5’-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 270:17513–17520. https://doi.org/10.1074/jbc.270.29.17513
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO (1994) Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta 1213:263–276. https://doi.org/10.1016/0005-2760(94)00082-4
Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90:207–258. https://doi.org/10.1152/physrev.00015.2009
Ahmad F, Woodgett JR (2020) Emerging roles of GSK-3alpha in pathophysiology: emphasis on cardio-metabolic disorders. Biochim Biophys Acta Mol Cell Res 1867:118616. S0167–4889(19)30224–1. https://doi.org/10.1016/j.bbamcr.2019.118616
Yamamoto T, Sano M (2022) Deranged myocardial fatty acid metabolism in heart failure. Int J Mol Sci 23. https://doi.org/10.3390/ijms23020996
Jiang M, Xie X, Cao F, Wang Y (2021) Mitochondrial metabolism in myocardial remodeling and mechanical unloading: implications for ischemic heart disease. Front Cardiovasc Med 8:789267. https://doi.org/10.3389/fcvm.2021.789267
Ahmad F, Singh AP, Tomar D, Rahmani M, Zhang Q, Woodgett JR, Tilley DG, Lal H, Force T (2019) Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload. J Mol Cell Cardiol 130:65–75. https://doi.org/10.1016/j.yjmcc.2019.03.020
Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E et al (2014) Cardiac fibroblast glycogen synthase kinase-3beta regulates ventricular remodeling and dysfunction in ischemic heart. Circulation 130:419–430. https://doi.org/10.1161/CIRCULATIONAHA.113.008364
Israeli-Rosenberg S, Manso AM, Okada H, Ross RS (2014) Integrins and integrin-associated proteins in the cardiac myocyte. Circ Res 114:572–586. https://doi.org/10.1161/CIRCRESAHA.114.301275
Diguet N, Trammell SAJ, Tannous C, Deloux R, Piquereau J, Mougenot N, Gouge A, Gressette M, Manoury B, Blanc J et al (2018) Nicotinamide riboside preserves cardiac function in a mouse model of dilated cardiomyopathy. Circulation 137:2256–2273. https://doi.org/10.1161/CIRCULATIONAHA.116.026099
Ahmad F, Tomar D, Aryal ACS, Elmoselhi AB, Thomas M, Elrod JW, Tilley DG, Force T (2020) Nicotinamide riboside kinase-2 alleviates ischemia-induced heart failure through P38 signaling. Biochim Biophys Acta Mol Basis Dis 1866:165609. https://doi.org/10.1016/j.bbadis.2019.165609
Shahzadi SK, Marzook H, Qaisar R, Ahmad F (2022) Nicotinamide riboside kinase-2 inhibits JNK pathway and limits dilated cardiomyopathy in mice with chronic pressure overload. Clin Sci (Lond) 136:181–196. https://doi.org/10.1042/CS20210964
Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, Song J, Yuan A, Shanmughapriya S, Guo Y et al (2016) Loss of adult cardiac myocyte GSK-3 leads to mitotic catastrophe resulting in fatal dilated cardiomyopathy. Circ Res 118:1208–1222. https://doi.org/10.1161/CIRCRESAHA.116.308544
Zhou J, Ahmad F, Lal H, Force T (2016) Response by Zhou et al to letter regarding article, “loss of adult cardiac myocyte gsk-3 leads to mitotic catastrophe resulting in fatal dilated cardiomyopathy.” Circ Res 119:e29–e30. https://doi.org/10.1161/CIRCRESAHA.116.309093
Lal H, Zhou J, Ahmad F, Zaka R, Vagnozzi RJ, Decaul M, Woodgett J, Gao E, Force T (2012) Glycogen synthase kinase-3alpha limits ischemic injury, cardiac rupture, post-myocardial infarction remodeling and death. Circulation 125:65–75. https://doi.org/10.1161/CIRCULATIONAHA.111.050666
Ahmad F, Lal H, Zhou J, Vagnozzi RJ, Yu JE, Shang X, Woodgett JR, Gao E, Force T (2014) Cardiomyocyte-specific deletion of gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J Am Coll Cardiol 64:696–706. https://doi.org/10.1016/j.jacc.2014.04.068
Khan AA, Gul MT, Karim A, Ranade A, Azeem M, Ibrahim Z, Ramachandran G, Nair VA, Ahmad F, Elmoselhi A et al (2022) Mitigating sarcoplasmic reticulum stress limits disuse-induced muscle loss in hindlimb unloaded mice. NPJ Microgravity 8:24. https://doi.org/10.1038/s41526-022-00211-w
Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16:284–287. https://doi.org/10.1089/omi.2011.0118
Gupte M, Lal H, Ahmad F, Sawyer DB, Hill MF (2017) Chronic neuregulin-1beta treatment mitigates the progression of postmyocardial infarction heart failure in the setting of type 1 diabetes mellitus by suppressing myocardial apoptosis, fibrosis, and key oxidant-producing enzymes. J Card Fail 23:887–899. https://doi.org/10.1016/j.cardfail.2017.08.456
Yusuf AM, Qaisar R, Al-Tamimi AO, Jayakumar MN, Woodgett JR, Koch WJ, Ahmad F (2022) Cardiomyocyte-GSK-3beta deficiency induces cardiac progenitor cell proliferation in the ischemic heart through paracrine mechanisms. J Cell Physiol 237:1804–1817. https://doi.org/10.1002/jcp.30644
Sharaf BM, Giddey AD, Alniss H, Al-Hroub HM, El-Awady R, Mousa M, Almehdi A, Soares NC, Semreen MH (2022) Untargeted metabolomics of breast cancer cells MCF-7 and SkBr 3 treated with tamoxifen/trastuzumab. Cancer Genomics Proteomics 19:79–93. https://doi.org/10.21873/cgp.20305
Pang Z, Chong J, Zhou G, de Lima Morais DA, Chang L, Barrette M, Gauthier C, Jacques PE, Li S, Xia J (2021) MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 49:W388–W396. https://doi.org/10.1093/nar/gkab382
Talman V, Ruskoaho H (2016) Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res 365:563–581. https://doi.org/10.1007/s00441-016-2431-9
Zuurbier CJ, Bertrand L, Beauloye CR, Andreadou I, Ruiz-Meana M, Jespersen NR, Kula-Alwar D, Prag HA, Eric Botker H, Dambrova M et al (2020) Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J Cell Mol Med 24:5937–5954. https://doi.org/10.1111/jcmm.15180
Chen C, Li R, Ross RS, Manso AM (2016) Integrins and integrin-related proteins in cardiac fibrosis. J Mol Cell Cardiol 93:162-174. S0022-2828(15)30115-2. https://doi.org/10.1016/j.yjmcc.2015.11.010
Schwartz MA, Ginsberg MH (2002) Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4:E65-68. https://doi.org/10.1038/ncb0402-e65
Schroer AK, Merryman WD (2015) Mechanobiology of myofibroblast adhesion in fibrotic cardiac disease. J Cell Sci 128:1865–1875. https://doi.org/10.1242/jcs.162891
Brancaccio M, Fratta L, Notte A, Hirsch E, Poulet R, Guazzone S, De Acetis M, Vecchione C, Marino G, Altruda F et al (2003) Melusin, a muscle-specific integrin β1-interacting protein, is required to prevent cardiac failure in response to chronic pressure overload. Nat Med 9:68–75
Su Z, Liu Y, Zhang H (2021) Adaptive cardiac metabolism under chronic hypoxia: mechanism and clinical implications. Front Cell Dev Biol 9:625524. https://doi.org/10.3389/fcell.2021.625524
Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC (2006) Trans fatty acids and cardiovascular disease. N Engl J Med 354:1601–1613. https://doi.org/10.1056/NEJMra054035
Marin-Garcia J, Goldenthal MJ (2002) Fatty acid metabolism in cardiac failure: biochemical, genetic and cellular analysis. Cardiovasc Res 54:516–527. https://doi.org/10.1016/s0008-6363(01)00552-1
Wang W, Ledee D (2021) ACAA2 is a ligand-dependent coactivator for thyroid hormone receptor beta1. Biochem Biophys Res Commun 576:15–21. https://doi.org/10.1016/j.bbrc.2021.08.073
Martines AMF, van Eunen K, Reijngoud DJ, Bakker BM (2017) The promiscuous enzyme medium-chain 3-keto-acyl-CoA thiolase triggers a vicious cycle in fatty-acid beta-oxidation. PLoS Comput Biol 13:e1005461. https://doi.org/10.1371/journal.pcbi.1005461
Liang X, Zhu D, Schulz H (1999) Delta 3,5,7, Delta2,4,6-trienoyl-CoA isomerase, a novel enzyme that functions in the beta-oxidation of polyunsaturated fatty acids with conjugated double bonds. J Biol Chem 274:13830–13835. https://doi.org/10.1074/jbc.274.20.13830
Mao X, Huang D, Rao C, Du M, Liang M, Li F, Liu B, Huang K (2020) Enoyl coenzyme A hydratase 1 combats obesity and related metabolic disorders by promoting adipose tissue browning. Am J Physiol Endocrinol Metab 318:E318–E329. https://doi.org/10.1152/ajpendo.00424.2019
Rai A, Kumar V, Jerath G, Kartha CC, Ramakrishnan V (2021) Mapping drug-target interactions and synergy in multi-molecular therapeutics for pressure-overload cardiac hypertrophy. NPJ Syst Biol Appl 7:11. https://doi.org/10.1038/s41540-021-00171-z
Abdulhag UN, Soiferman D, Schueler-Furman O, Miller C, Shaag A, Elpeleg O, Edvardson S, Saada A (2015) Mitochondrial complex IV deficiency, caused by mutated COX6B1, is associated with encephalomyopathy, hydrocephalus and cardiomyopathy. Eur J Hum Genet 23:159–164. https://doi.org/10.1038/ejhg.2014.85
McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB (2018) Cardiovascular metabolomics. Circ Res 122:1238–1258. https://doi.org/10.1161/CIRCRESAHA.117.311002
de Couto G, Ouzounian M, Liu PP (2010) Early detection of myocardial dysfunction and heart failure. Nat Rev Cardiol 7:334–344. https://doi.org/10.1038/nrcardio.2010.51
Bathaie SZ, Ashrafi M, Azizian M, Tamanoi F (2017) Mevalonate pathway and human cancers. Curr Mol Pharmacol 10:77–85. https://doi.org/10.2174/1874467209666160112123205
Xu H, Shen Y, Liang C, Wang H, Huang J, Xue P, Luo M (2021) Inhibition of the mevalonate pathway improves myocardial fibrosis. Exp Ther Med 21:224. https://doi.org/10.3892/etm.2021.9655
Goldstein JL, Brown MS (1990) Regulation of the mevalonate pathway. Nature 343:425–430. https://doi.org/10.1038/343425a0
Yang Y, Rong X, Lv X, Jiang W, Lai D, Xu S, Fu G (2017) Inhibition of mevalonate pathway prevents ischemia-induced cardiac dysfunction in rats via RhoA-independent signaling pathway. Cardiovasc Ther 35. https://doi.org/10.1111/1755-5922.12285
Howes LG, Hodsman GP, Rowe PR, Sumithran E, Johnston CI (1989) Cardiac 3,4-dihydroxyphenylethylene glycol (DHPG) and catecholamine levels during perindopril therapy of chronic left ventricular failure in rats. J Auton Pharmacol 9:15–21. https://doi.org/10.1111/j.1474-8673.1989.tb00192.x
Bohner H, Janiak PS, Nitsche V, Eichinger A, Schutz H (1997) Relative bioavailability of different butamirate citrate preparations after single dose oral administration to 18 healthy volunteers. Int J Clin Pharmacol Ther 35:117–122
Cao JY, Parker B, Koay YC, Lal S, O’Sullivan JF (2018) Identification of new pathways in human ischemic myocardium. Circulation 138:A12816
Krylova IB, Selina EN, Bulion VV, Rodionova OM, Evdokimova NR, Belosludtseva NV, Shigaeva MI, Mironova GD (2021) Uridine treatment prevents myocardial injury in rat models of acute ischemia and ischemia/reperfusion by activating the mitochondrial ATP-dependent potassium channel. Sci Rep 11:16999. https://doi.org/10.1038/s41598-021-96562-7
Guo M, Fan X, Tuerhongjiang G, Wang C, Wu H, Lou B, Wu Y, Yuan Z, She J (2021) Targeted metabolomic analysis of plasma fatty acids in acute myocardial infarction in young adults. Nutr Metab Cardiovasc Dis 31:3131–3141. https://doi.org/10.1016/j.numecd.2021.06.024
Renaud SC (1992) What is the epidemiologic evidence for the thrombogenic potential of dietary long-chain fatty acids? Am J Clin Nutr 56:823S-824S. https://doi.org/10.1093/ajcn/56.4.823s
Zhuang L, Mao Y, Liu Z, Li C, Jin Q, Lu L, Tao R, Yan X, Chen K (2021) FABP3 deficiency exacerbates metabolic derangement in cardiac hypertrophy and heart failure via pparalpha pathway. Front Cardiovasc Med 8:722908. https://doi.org/10.3389/fcvm.2021.722908
Furuhashi M, Hotamisligil GS (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat Rev Drug Discov 7:489–503. https://doi.org/10.1038/nrd2589
Lee SM, Lee SH, Jung Y, Lee Y, Yoon JH, Choi JY, Hwang CY, Son YH, Park SS, Hwang GS et al (2020) FABP3-mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat Commun 11:5661. https://doi.org/10.1038/s41467-020-19501-6
Varrone F, Gargano B, Carullo P, Di Silvestre D, De Palma A, Grasso L, Di Somma C, Mauri P, Benazzi L, Franzone A et al (2013) The circulating level of FABP3 is an indirect biomarker of microRNA-1. J Am Coll Cardiol 61:88–95. https://doi.org/10.1016/j.jacc.2012.08.1003
Li A, Zheng N, Ding X (2022) Mitochondrial abnormalities: a hub in metabolic syndrome-related cardiac dysfunction caused by oxidative stress. Heart Fail Rev 27:1387–1394. https://doi.org/10.1007/s10741-021-10109-6
Rose BA, Force T, Wang Y (2010) Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev 90:1507–1546. https://doi.org/10.1152/physrev.00054.2009
Li J, Rao H, Burkin D, Kaufman SJ, Wu C (2003) The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition. Dev Biol 261:209–219
McNally EM, Mestroni L (2017) Dilated Cardiomyopathy: genetic determinants and mechanisms. Circ Res 121:731–748. https://doi.org/10.1161/CIRCRESAHA.116.309396
Gomez-Sanchez R, Yakhine-Diop SM, Bravo-San Pedro JM, Pizarro-Estrella E, Rodriguez-Arribas M, Climent V, Martin-Cano FE, Gonzalez-Soltero ME, Tandon A, Fuentes JM et al (2016) PINK1 deficiency enhances autophagy and mitophagy induction. Mol Cell Oncol 3:e1046579. https://doi.org/10.1080/23723556.2015.1046579
Kuhn C, Menke M, Senger F, Mack C, Dierck F, Hille S, Schmidt I, Brunke G, Bunger P, Schmiedel N et al (2021) FYCO1 Regulates Cardiomyocyte Autophagy and Prevents Heart Failure Due to Pressure Overload In Vivo. JACC Basic Transl Sci 6:365–380. https://doi.org/10.1016/j.jacbts.2021.01.001
Funding
The work was supported by Collaborative (22010901112) and Targeted (1801090144), research grants from the University of Sharjah to Firdos Ahmad. R.H. is funded by ASPIRE “Abu Dhabi Precision Medicine ARI” (VRI-20-10) grant.
Author information
Authors and Affiliations
Contributions
HM performed experiments, collected data, and helped with manuscript writing, AG performed experiments and collected data, MAS, KP, MHS, and RH helped with data analysis and interpretation, DT and NCS helped with experiments and data analysis, RQ helped with data analysis and interpretation and manuscript writing, and FA designed the study, acquired funding, supervised the project, performed data analysis and interpretation, and wrote first draft and revised the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University Medical Center approved all animal procedures and treatments.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marzook, H., Gupta, A., Tomar, D. et al. Nicotinamide riboside kinase-2 regulates metabolic adaptation in the ischemic heart. J Mol Med 101, 311–326 (2023). https://doi.org/10.1007/s00109-023-02296-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-023-02296-6