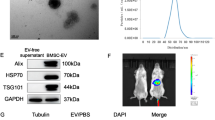
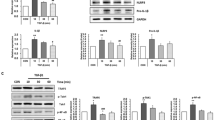
Abstract
TGF‐β1 is the strongest cytokine known to promote liver fibrosis. It has been previously demonstrated that the activation of TGF‐β1 initiates a temporary collagen accumulation program, which is important for wound repair in several organs. Furthermore, temporary extracellular matrix enhancement often leads to progressive fibrosis, which is accountable for cases of severe morbidity and mortality worldwide. However, its action mechanism has not been fully explored. It was previously reported that UCA1 could promote its occurrence and development in various tumors. Importantly, it was reported that TGF‐β1 could activate the expression of UCA1 in liver cancer, gastric cancer, and breast cancer. However, the role of UCA1 in organ fibrosis, including liver fibrosis, remains unreported. The present study reported for the first time that TGF‐β1/Smad3 could promote liver fibrosis by upregulating UCA1, which further affected DKK1 and collagen, such as COL1A1, COL1A2, and COL3A1. Meanwhile, UCA1 could competitively bind with miR18a to stabilize Smad3 to constitute a positive feedback pathway, which played a significant role in the promotion of liver fibrosis. Altogether, the present study provides a theoretical basis for devising promising treatment strategies for liver fibrosis.
Key messages
-
UCA1 was found to promote the progression of liver fibrosis in vitro.
-
UCA1 is regulated by TGF-β1 and promotes liver fibrosis through the canonical Smad pathway.
-
UCA1 can competitively bind with miR18a, promote liver fibrosis by stabilizing Smad3, and form a UCA1-miR18a/Smad3 positive feedback.
-
UCA1 binds EZH2 to inhibit the DKK1 expression and promote liver fibrosis.








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Song L, Chen TY, Zhao XJ et al (2019) Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. Br J Pharmacol 176:1619–1634. https://doi.org/10.1111/bph.14573
Boutanquoi PM, Burgy O, Beltramo G et al (2020) TRIM33 prevents pulmonary fibrosis by impairing TGF-β1 signalling. Eur Respir J 11:1901346. https://doi.org/10.1183/13993003.01346-2019
Loboda A, Sobczak M, Jozkowicz A et al (2016) TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediators Inflamm 2016:8319283. https://doi.org/10.1155/2016/8319283
Henderson NC, Rieder F, Wynn TA (2020) Fibrosis: from mechanisms to medicines. Nature 587:555–566. https://doi.org/10.1038/s41586-020-2938-9
Wrana JL, Attisano L, Wieser R et al (1994) Mechanism of activation of the TGF-β receptor. Nature 370:341–347. https://doi.org/10.1038/370341a0
Kim KK, Sheppard D, Chapman HA (2018) TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 10:a022293. https://doi.org/10.1101/cshperspect.a022293
Wei Y, Kim TJ, Peng DH et al (2017) Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis. J Clin Invest 127:3675–3688. https://doi.org/10.1172/JCI94624
Zhao X, Kwan JYY, Yip K et al (2020) Targeting metabolic dysregulation for fibrosis therapy. Nat Rev Drug Discov 19:57–75. https://doi.org/10.1038/s41573-019-0040-5
Hu ML, Wang XY, Chen WM (2018) TGF-β1 upregulates the expression of lncRNA UCA1 and its downstream HXK2 to promote the growth of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci 22:4846–4854. https://doi.org/10.26355/eurrev_201808_15620
Zuo ZK, Gong Y, Chen XH et al (2017) TGFβ1-induced LncRNA UCA1 upregulation promotes gastric cancer invasion and migration. DNA Cell Biol 36:159–167. https://doi.org/10.1089/dna.2016.3553
Li GY, Wang W, Sun JY et al (2018) Long non-coding RNAs AC026904.1 and UCA1: a “one-two punch” for TGF-β-induced SNAI2 activation and epithelial-mesenchymal transition in breast cancer. Theranostics 8:2846–2861. https://doi.org/10.7150/thno.23463
Bian EB, Wang YY, Yang Y et al (2017) Hotair facilitates hepatic stellate cells activation and fibrogenesis in the liver. Biochim Biophys Acta Mol Basis Dis 1863:674–686. https://doi.org/10.1016/j.bbadis.2016.12.009
Yu F, Chen B, Fan X et al (2017) Epigenetically-regulated microRNA-9-5p suppresses the activation of hepatic stellate cells via TGFBR1 and TGFBR2. Cell Physiol Biochem 43:2242–2252. https://doi.org/10.1159/000484303
Yang Y, Chen XX, Li WX et al (2017) EZH2-mediated repression of Dkk1 promotes hepatic stellate cell activation and hepatic fibrosis. J Cell Mol Med 21:2317–2328. https://doi.org/10.1111/jcmm.13153
Xu T, Yan S, Wang M, Jiang L et al (2020) LncRNA UCA1 induces acquired resistance to gefitinib by epigenetically silencing CDKN1A expression in non-small-cell lung cancer. Front Oncol 10:656. https://doi.org/10.3389/fonc.2020.00656
Hu JJ, Song W, Zhang SD et al (2016) HBx-upregulated lncRNA UCA1 promotes cell growth and tumorigenesis by recruiting EZH2 and repressing p27Kip1/CDK2 signaling. Sci Rep 6:23521. https://doi.org/10.1038/srep23521
He X, Wang J, Chen J et al (2019) lncRNA UCA1 predicts a poor prognosis and regulates cell proliferation and migration by repressing p21 and SPRY1 expression in GC. Mol Ther Nucleic Acids 18:605–616. https://doi.org/10.1016/j.omtn.2019.09.024
Cai Q, Jin L, Wang S et al (2017) Long non-coding RNA UCA1 promotes gallbladder cancer progression by epigenetically repressing p21 and E-cadherin expression. Oncotarget 8:47957–47968. https://doi.org/10.18632/oncotarget
Thiele BJ, Doller A, Kähne T et al (2004) RNA-binding proteins heterogeneous nuclear ribonucleoprotein A1, E1, and K are involved in post-transcriptional control of collagen I and III synthesis. Circ Res 95:1058–1066. https://doi.org/10.1161/01.RES.0000149166.33833.08
Roderburg C, Urban GW, Bettermann K et al (2011) Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53:209–218. https://doi.org/10.1002/hep.23922
Sekiya Y, Ogawa T, Yoshizato K et al (2011) Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun 412:74–79. https://doi.org/10.1016/j.bbrc.2011.07.041
Huang YH, Yang YL, Wang FS (2018) The role of miR-29a in the regulation, function, and signaling of liver fibrosis. Int J Mol Sci 19:1889. https://doi.org/10.3390/ijms19071889
Wang X, He Y, Mackowiak B et al (2021) MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut 70:784–795. https://doi.org/10.1136/gutjnl-2020-322526
Li X, Wu Y, Liu A et al (2016) Long non-coding RNA UCA1 enhances tamoxifen resistance in breast cancer cells through a miR-18a-HIF1α feedback regulatory loop. Tumour Biol 37:14733–14743. https://doi.org/10.1007/s13277-016-5348-8
Kolenda T, Guglas K, Kopczyńska M et al (2020) Good or not good: role of miR-18a in cancer biology. Rep Pract Oncol Radiother 25:808–819. https://doi.org/10.1016/j.rpor.2020.07.006
Xu P, Li Z, Wang Y, Yu X et al (2020) miR-18a Contributes to preeclampsia by downregulating Smad2 (full length) and reducing TGF-β signaling. Mol Ther Nucleic Acids 22:542–556. https://doi.org/10.1016/j.omtn.2020.09.019
Lian C, Tao T, Su P et al (2020) Targeting miR-18a sensitizes chondrocytes to anticytokine therapy to prevent osteoarthritis progression. Cell Death Dis 11:947. https://doi.org/10.1038/s41419-020-03155-9
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338. https://doi.org/10.1038/nrneph.2016.48
Hata A, Chen YG (2016) TGF-β signaling from receptors to Smads. Cold Spring Harb Perspect Biol 8:a022061. https://doi.org/10.1101/cshperspect.a022061
Zhang K, Han X, Zhang Z et al (2017) The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat Commun 8:144. https://doi.org/10.1038/s41467-017-00204-4
Klavdianou K, Liossis SN, Daoussis D (2017) Dkk1: a key molecule in joint remodelling and fibrosis. Mediterr J Rheumatol 28:174–182. https://doi.org/10.31138/mjr.28.4.174
Akhmetshina A, Palumbo K, Dees C et al (2012) Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun 3:735. https://doi.org/10.1038/ncomms1734
Liu Z, Wang Y, Yuan S et al (2021) Regulatory role of long non-coding RNA UCA1 in signaling pathways and its clinical applications. Oncol Lett 21:404. https://doi.org/10.3892/ol.2021.12665
Guo X, Zhang Y, Mayakonda A et al (2018) ARID1A and CEBPα cooperatively inhibit UCA1 transcription in breast cancer. Oncogene 37:5939–5951. https://doi.org/10.1038/s41388-018-0371-4
Wu W, Zhang S, Li X et al (2013) Ets-2 regulates cell apoptosis via the Akt pathway, through the regulation of urothelial cancer associated 1, a long non-coding RNA, in bladder cancer cells. PLoS ONE 8:e73920. https://doi.org/10.1371/journal.pone.0073920
Wang ZQ, Cai Q, Hu L et al (2017) Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis 8:e2839. https://doi.org/10.1038/cddis.2017.143
Cui X, Zhao C, Yao X et al (2018) SND1 acts as an anti-apoptotic factor via regulating the expression of lncRNA UCA1 in hepatocellular carcinoma. RNA Biol 15:1364–1375. https://doi.org/10.1080/15476286.2018.1534525
He H, Mennone A, Boyer JL et al (2011) Combination of retinoic acid and ursodeoxycholic acid attenuates liver injury in bile duct-ligated rats and human hepatic cells. Hepatology 53:548–557. https://doi.org/10.1002/hep.24047
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers 81770283, 82070302, 81902018, 81903001, and 81772926); Clinical Medical Research Center of Peritoneal Cancer of Wuhan (grant number 2015060911020462); Natural Science Foundation of Hubei Province (grant numbers 2019CFB109 and 2020CFB708); Technology and Innovation Seed Fund, Zhongnan Hospital of Wuhan University (grant number znpy2018004); and Medical Sci-Tech Innovation Platform, Zhongnan Hospital of Wuhan University (grant number PTXM2021022).
Author information
Authors and Affiliations
Contributions
Conception and design: ZS.Y. and B.L.; development of methodology: ZS.Y., H.Z., and MH.Y.; acquisition of data: ZS.Y., MH.Y., ZX.C., and P.J.; analysis of data: ZS.Y., MH.F., B.L., and ZS.L.; writing, review, and/or revision of the manuscript: ZS.Y., H.Z., B.L., MH.F., and ZS.L. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The tissues collected in this paper have passed the ethical approval of Wuhan University Zhongnan Hospital. This study conforms to the ethical guidelines of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, Z., Zhang, H., Yin, M. et al. TGF-β1/Smad3 upregulates UCA1 to promote liver fibrosis through DKK1 and miR18a. J Mol Med 100, 1465–1478 (2022). https://doi.org/10.1007/s00109-022-02248-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02248-6