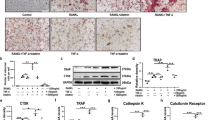
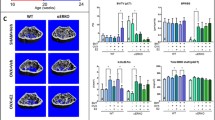
Abstract
Under normal conditions, the human body employs the synergistic action of osteoblasts and osteoclasts to maintain a dynamic balance between bone formation and resorption. Bone homeostasis plays a very important role in the process of bone formation. Various bone diseases can occur if bone homeostasis is disrupted. In this study, the serum estrogen levels were significantly increased in the granulin (GRN)–deficient mice and PGRN regulates the binding of estrogen and estrogen receptor α (ERα) and then affects estrogen’s ability to regulate bone formation and resorption. In addition, this study also explored the role that PGRN plays in regulating bone homeostasis by affecting the binding of estrogen and estrogen receptors through the protein kinase R-like endoplasmic reticulum kinase/phosphorylation of the eukaryotic initiation factor 2 signaling pathway. In summary, we confirmed the important role of PGRN in regulating the estrogen (E2)/ERα signal in maintaining bone homeostasis. Our findings may provide a new strategy for the treatment of osteoporosis and maintaining bone homeostasis.
Key messages
-
PGRN is a molecular regulator of the binding of E2 and ERα signal in maintaining bone homeostasis.
-
PGRN plays in regulating bone homeostasis through the PERK/p-eIF2α signaling pathway.
-
The best therapeutic effect of PGRN in osteoporosis is associated with different concentration of E2.









Similar content being viewed by others
Authors’ information
We are committed to studying ER stress, autophagy, and their relation to bone development and bone-associated diseases. Y.Y.Y, N.B.F, Y.M.P., and G.N.N. were doctoral students; L.L. was a master’s student; M.T.F. was a postdoctoral researcher; R.J., X.L.L, and F.J.G. were teachers and researchers.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- PGRN:
-
Progranulin
- GRN:
-
Granulin
- ERα:
-
Estrogen receptor α
- PERK:
-
Protein kinase RNA (PKR)–like ER kinase
- p-eIF2α:
-
Phosphorylation of eukaryotic initiation factor 2 α
- E2:
-
Estrogen
- OVX:
-
Ovariectomy
- XBP1:
-
X-box binding protein 1
- ATF4:
-
Activating transcription factor 4
- ER:
-
Endoplasmic reticulum
- BMP-2:
-
Bone morphogenetic protein-2
- RUNX2:
-
Runt-related transcription factor 2
- OPG:
-
Osteoprotegerin
- DMP1:
-
Dentin matrix acidic phosphoprotein 1
- NFATc1:
-
Nuclear factor of activated T cells, cytoplasmic 1
- ACP5:
-
Acid phosphatase 5
- CTSK:
-
Cathepsin K
- RANK:
-
Receptor activator of NF-κB
- RANKL:
-
RANK ligand
- M-CSF:
-
Macrophage colony-stimulating factor
- TRAP:
-
Tartrate-resistant acid phosphatase
- VMD:
-
Visual molecular dynamics
- CRISPR-Cas9:
-
Clustered regularly interspaced short palindromic repeats-associated protein 9
References
Ensrud KE, Crandall CJ (2017) Osteoporosis. Ann Intern Med 167(3):Itc17-itc32
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19(2):179–192
Reid IR (2020) A broader strategy for osteoporosis interventions. Nat Rev Endocrinol 16(6):333–339
Söreskog E, Lindberg I, Kanis JA et al (2021) Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int 32(3):585–594
Leder BZ (2017) Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep 15(2):110–119
Chen LR, Hou PH, Chen KH (2019) Nutritional Support and physical modalities for people with osteoporosis: current opinion. Nutrients 11(12)
Xu R, Yallowitz A, Qin A et al (2018) Targeting skeletal endothelium to ameliorate bone loss. Nat Med 24(6):823–833
Michalski MN, McCauley LK (2017) Macrophages and skeletal health. Pharmacol Ther 174:43–54
Bandeira L, Bilezikian JP (2017) Novel therapies for postmenopausal osteoporosis. Endocrinol Metab Clin North Am 46(1):207–219
Black DM, Rosen CJ (2016) Clinical Practice. Postmenopausal Osteoporosis N Engl J Med 374(3):254–262
Lanyan A, Marques-Vidal P, Gonzalez-Rodriguez E et al (2020) Postmenopausal women with osteoporosis consume high amounts of vegetables but insufficient dairy products and calcium to benefit from their virtues: the CoLaus/OsteoLaus cohort. Osteoporos Int 31(5):875–886
Hwang WJ, Lee TY, Kim NS et al (2020) The role of estrogen receptors and their signaling across psychiatric disorders. Int J Mol Sci 22(1)
D’Alton S, Lewis J (2014) Understanding the role of progranulin in Alzheimer’s disease. Nat Med 20(10):1099–1100
Xu HM, Tan L, Wan Y et al (2017) PGRN Is associated with late-onset Alzheimer’s disease: a Case-Control Replication Study and Meta-analysis. Mol Neurobiol 54(2):1187–1195
Perry DC, Lehmann M, Yokoyama JS et al (2013) Progranulin mutations as risk factors for Alzheimer disease. JAMA Neurol 70(6):774–778
Klein ZA, Takahashi H, Ma M et al (2017) Loss of TMEM106B ameliorates lysosomal and frontotemporal dementia-related phenotypes in progranulin-deficient mice. Neuron 95(2):281–96.e6
Tavares TP, Mitchell DGV, Coleman KK et al (2020) Early symptoms in symptomatic and preclinical genetic frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry 91(9):975–984
Mateo I, González-Aramburu I, Pozueta A et al (2013) Reduced serum progranulin level might be associated with Parkinson’s disease risk. Eur J Neurol 20(12):1571–1573
Nicoletto BB, Canani LH (2015) The role of progranulin in diabetes and kidney disease. Diabetol Metab Syndr. 7:117
Zhou D, Zhou M, Wang Z et al (2019) Progranulin alleviates podocyte injury via regulating CAMKK/AMPK-mediated autophagy under diabetic conditions. J Mol Med (Berl) 97(11):1507–1520
Laudisi F, Cherubini F, Di Grazia A et al (2019) Progranulin sustains STAT3 hyper-activation and oncogenic function in colorectal cancer cells. Mol Oncol 13(10):2142–2159
Yue S, Ye X, Zhou T et al (2021) PGRN(-/-) TAMs-derived exosomes inhibit breast cancer cell invasion and migration and its mechanism exploration. Life Sci 264:118687
Voshtani R, Song M, Wang H et al (2019) Progranulin promotes melanoma progression by inhibiting natural killer cell recruitment to the tumor microenvironment. Cancer Lett 465:24–35
Jian J, Konopka J, Liu C (2013) Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol 93(2):199–208
Zou S, Luo Q, Song Z et al (2017) Contribution of progranulin to protective lung immunity during bacterial pneumonia. J Infect Dis 215(11):1764–1773
Feng JQ, Guo FJ, Jiang BC et al (2010) Granulin epithelin precursor: a bone morphogenic protein 2-inducible growth factor that activates Erk1/2 signaling and JunB transcription factor in chondrogenesis. Faseb j 24(6):1879–1892
Zhang Y, Lin J, Wei F (2015) The function and roles of ADAMTS-7 in inflammatory diseases. Mediators Inflamm 2015:801546
Wei JL, Fu W, Ding YJ et al (2017) Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis Res Ther 19(1):280
Tang W, Lu Y, Tian QY et al (2011) The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice. Science 332(6028):478–484
Savić N, Schwank G (2016) Advances in therapeutic CRISPR/Cas9 genome editing. Transl Res 168:15–21
Zhang F, Wen Y, Guo X (2014) CRISPR/Cas9 for genome editing: progress, implications and challenges. Hum Mol Genet 23(R1):R40–R46
Komori T (2019) Regulation of proliferation, differentiation and functions of osteoblasts by Runx2. Int J Mol Sci 20(7)
Komori T (2020) Molecular mechanism of Runx2-dependent bone development. Mol Cells 43(2):168–175
Reid P, Holen I (2009) Pathophysiological roles of osteoprotegerin (OPG). Eur J Cell Biol 88(1):1–17
Xue H, Niu P, Liu Y et al (2020) Glycosylation of DMP1 promotes bone reconstruction in long bone defects. Biochem Biophys Res Commun 526(4):1125–1130
Zhao Q, Wang X, Liu Y et al (2010) NFATc1: functions in osteoclasts. Int J Biochem Cell Biol 42(5):576–579
Ren X, Shan WH, Wei LL et al (2018) ACP5: Its structure, distribution, regulation and novel functions. Anticancer Agents Med Chem 18(8):1082–1090
Vervloet MG, Brandenburg VM (2017) Circulating markers of bone turnover. J Nephrol 30(5):663–670
Kozawa E, Nishida Y, Cheng XW et al (2012) Osteoarthritic change is delayed in a Ctsk-knockout mouse model of osteoarthritis. Arthritis Rheum 64(2):454–464
Levin VA, Jiang X, Kagan R (2018) Estrogen therapy for osteoporosis in the modern era. Osteoporos Int 29(5):1049–1055
Tella SH, Gallagher JC (2014) Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol 142:155–70
Gavali S, Gupta MK, Daswani B et al (2019) Estrogen enhances human osteoblast survival and function via promotion of autophagy. Biochim Biophys Acta Mol Cell Res 1866(9):1498–1507
Wu GJ, Chen JT, Lin PI et al (2020) Inhibition of the estrogen receptor alpha signaling delays bone regeneration and alters osteoblast maturation, energy metabolism, and angiogenesis. Life Sci 258:118195
Xi G, Demambro VE, D'Costa S et al (2020) Estrogen stimulation of pleiotrophin enhances osteoblast differentiation and maintains bone mass in IGFBP-2 null mice. Endocrinology 161(4)
Khalid AB, Krum SA (2016) Estrogen receptors alpha and beta in bone. Bone 87:130–5
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23(11):576–581
Bezamat M, Deeley K, Khaliq S et al (2019) Are mTOR and endoplasmic reticulum stress pathway genes associated with oral and bone diseases? Caries Res 53(3):235–241
Li H, Li D, Ma Z et al (2018) Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 14(10):1726–1741
Li J, Yang S, Li X et al (2017) Role of endoplasmic reticulum stress in disuse osteoporosis. Bone 97:2–14
Wu G, Xu R, Zhang P et al (2018) Estrogen regulates stemness and senescence of bone marrow stromal cells to prevent osteoporosis via ERβ-SATB2 pathway. J Cell Physiol 233(5):4194–4204
Cui Y, Hettinghouse A, Liu CJ (2019) Progranulin: a conductor of receptors orchestra, a chaperone of lysosomal enzymes and a therapeutic target for multiple diseases. Cytokine Growth Factor Rev 45:53–64
Oh J, Kim JY, Kim HS et al (2015) Progranulin and a five transmembrane domain-containing receptor-like gene are the key components in receptor activator of nuclear factor κB (RANK)-dependent formation of multinucleated osteoclasts. J Biol Chem 290(4):2042–2052
Noguchi T, Ebina K, Hirao M et al (2015) Progranulin plays crucial roles in preserving bone mass by inhibiting TNF-α-induced osteoclastogenesis and promoting osteoblastic differentiation in mice. Biochem Biophys Res Commun 465(3):638–643
Wang L, Roth T, Nakamura MC et al (2019) Female-specific role of progranulin to suppress bone formation. Endocrinology 160(9):2024–2037
Acknowledgements
All experiments are completed on the experimental platform provided by the Core Facility of Development Biology of Basic Medical College (Chongqing Medical University).
Funding
Our experiments were financially supported by the Natural Science Foundation of China (No.81672209, No.81871769); Chongqing Science and Technology Bureau (cstc2020jcyj-msxmX0175); and Chongqing Human Resources and Social Security Bureau (2018–389).
Author information
Authors and Affiliations
Contributions
All authors participated in the design, interpretation of the studies and analysis of the data, and review of the manuscript; all authors approved the final version to be published. Y.Y.Y, N.B.F, L.L., Y.M.P., G.N.N., M.T.F., and R.J. conducted the experiments; N.B.F, Y.Y.Y, L.L., and F.J.G. analyzed and interpreted the data; Prof. Fengjin Guo designed the manuscript and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the final manuscript prior to submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved and granted by the Ethics Committee of Chongqing Medical University (March 23, 2021).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Y., Feng, N., liang, L. et al. Progranulin, a moderator of estrogen/estrogen receptor α binding, regulates bone homeostasis through PERK/p-eIF2 signaling pathway. J Mol Med 100, 1191–1207 (2022). https://doi.org/10.1007/s00109-022-02233-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-022-02233-z