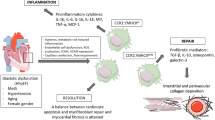
Abstract
Macrophages are integral components of the mammalian heart that show extensive expansion in response to various internal or external stimuli. After the onset of sustained pressure overload (PO), the accumulation of cardiac macrophages through local macrophage proliferation and monocyte migration has profound effects on the transition to cardiac hypertrophy and remodeling. In this review, we describe the heterogeneity and diversity of cardiac macrophages and summarize the current understanding of the important roles of macrophages in PO-induced cardiac remodeling. In addition, the possible mechanisms involved in macrophage modulation are also described. Finally, considering the significant effects of cardiac macrophages, we highlight their emerging role as therapeutic targets for alleviating pathological cardiac remodeling after PO.


Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- AAC:
-
Abdominal aortic constriction
- Ang II:
-
Angiotensin II
- CCL:
-
C-C motif chemokine ligand
- CCR:
-
C-C motif chemokine receptor
- CD:
-
Cluster of differentiation
- CM:
-
Cardiomyocyte
- CSF2:
-
Colony-stimulating factor 2
- CX3C:
-
C-X3-C motif chemokine
- CXCL:
-
Chemokine (C-X-C motif) ligand
- CXCR:
-
C-X-C motif chemokine receptor
- DNMT3A:
-
DNA methyltransferase 3 alpha
- GATA3:
-
GATA binding protein 3
- HF:
-
Heart failure
- HIF:
-
Hypoxia inducible factor
- HLA:
-
Human leukocyte antigen
- ICAM1:
-
Intercellular cell adhesion molecule 1
- IFN–γ:
-
Interferon gamma
- IM:
-
Interstitial macrophage
- KO:
-
Knockout
- LFA-1:
-
Lymphocyte function-associated antigen-1
- M-CSF:
-
Macrophage-colony stimulating factor
- MI:
-
Myocardial infarction
- MIF:
-
Macrophage migration inhibitory factor
- NLRP3:
-
NLR family pyrin domain containing 3
- PHD:
-
Prolyl hydroxylase domain
- PI3K:
-
Phosphatidylinositol 3-kinase
- PO:
-
Pressure overload
- RAS:
-
Renin-angiotensin system
- RHOA:
-
Ras homolog family member A
- SNP:
-
Single nucleotide polymorphism
- TAC:
-
Transverse aortic constriction
- TET2:
-
Tet methylcytosine dioxygenase 2
- TNFα:
-
Tumor necrosis factor α
- TRAF6:
-
TNF receptor-associated factor 6
- XCL:
-
Chemokine (X-C motif) ligand
- WT:
-
Wild type
References
Nakamura M, Sadoshima J (2018) Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol 15:387–407
Shimizu I, Minamino T (2016) Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 97:245–262
Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13:368–378
Schiattarella GG, Hill JA (2015) Inhibition of hypertrophy is a good therapeutic strategy in ventricular pressure overload. Circulation 131:1435–1447
Dadson K, Kovacevic V, Rengasamy P, Kim GH, Boo S, Li RK, George I, Schulze PC, Hinz B, Sweeney G (2016) Cellular, structural and functional cardiac remodelling following pressure overload and unloading. Int J Cardiol 216:32–42
Andreadou I, Cabrera-Fuentes HA, Devaux Y, Frangogiannis NG, Frantz S, Guzik T, Liehn EA, Gomes CPC, Schulz R, Hausenloy DJ (2019) Immune cells as targets for cardioprotection: new players and novel therapeutic opportunities. Cardiovasc Res 115:1117–1130
Zhang Y, Huang Z, Li H (2017) Insights into innate immune signalling in controlling cardiac remodelling. Cardiovasc Res 113:1538–1550
Latet SC, Hoymans VY, Van Herck PL, Vrints CJ (2015) The cellular immune system in the post-myocardial infarction repair process. Int J Cardiol 179:240–247
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA et al (2016) Revisiting cardiac cellular composition. Circ Res 118:400–409
Wang L, Yu P, Zhou B, Song J, Li Z, Zhang M, Guo G, Wang Y, Chen X, Han L, Hu S (2020) Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat Cell Biol 22:108–119
Kamo T, Akazawa H, Komuro I (2015) Cardiac nonmyocytes in the hub of cardiac hypertrophy. Circ Res 117:89–98
Frieler RA, Mortensen RM (2015) Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131:1019–1030
Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, Carriero R, Termanini A, Colombo FS, Jachetti E, Panico C, Faggian G, Fumero A, Torracca L, Molgora M, Cibella J, Pagiatakis C, Brummelman J, Alvisi G, Mazza EMC, Colombo MP, Lugli E, Condorelli G, Kallikourdis M (2019) Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation 140:2089–2107
Hulsmans M, Sam F, Nahrendorf M (2016) Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol 93:149–155
Kim AJ, Xu N, Umeyama K, Hulin A, Ponny SR, Vagnozzi RJ, Green EA, Hanson P, McManus BM, Nagashima H et al (2020) Deficiency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in Marfan syndrome. Circulation 141:132–146
Kim AJ, Xu N, Yutzey KE (2020) Macrophage lineages in heart valve development and disease. Cardiovasc Res. https://doi.org/10.1093/cvr/cvaa062
Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC (2018) The macrophage in cardiac homeostasis and disease JACC macrophage in CVD series (part 4). J Am Coll Cardiol 72:2213–2230
Honold L, Nahrendorf M (2018) Resident and monocyte-derived macrophages in cardiovascular disease. Circ Res 122:113–127
Glezeva N, Horgan S, Baugh JA (2015) Monocyte and macrophage subsets along the continuum to heart failure: misguided heroes or targetable villains? J Mol Cell Cardiol 89:136–145
Ren Z, Yu P, Li D, Li Z, Liao Y, Wang Y, Zhou B, Wang L (2020) Single-cell reconstruction of progression trajectory reveals intervention principles in pathological cardiac hypertrophy. Circulation 141:1704–1719
Kain D, Amit U, Yagil C, Landa N, Naftali-Shani N, Molotski N, Aviv V, Feinberg MS, Goitein O, Kushnir T, Konen E, Epstein FH, Yagil Y, Leor J (2016) Macrophages dictate the progression and manifestation of hypertensive heart disease. Int J Cardiol 203:381–395
Blériot C, Chakarov S, Ginhoux F (2020) Determinants of resident tissue macrophage identity and function. Immunity 52:957–970
Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC (2012) The three human monocyte subsets: implications for health and disease. Immunol Res 53:41–57
Patel AA, Zhang Y, Fullerton JN, Boelen L, Rongvaux A, Maini AA, Bigley V, Flavell RA, Gilroy DW, Asquith B, Macallan D, Yona S (2017) The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med 214:1913–1923
Thomas GD, Hamers AAJ, Nakao C, Marcovecchio P, Taylor AM, McSkimming C, Nguyen AT, McNamara CA, Hedrick CC (2017) Human blood monocyte subsets: a new gating strategy defined using cell surface markers identified by mass cytometry. Arterioscler Thromb Vasc Biol 37:1548–1558
Hamers AAJ, Dinh HQ, Thomas GD, Marcovecchio P, Blatchley A, Nakao CS, Kim C, McSkimming C, Taylor AM, Nguyen AT, McNamara CA, Hedrick CC (2019) Human monocyte heterogeneity as revealed by high-dimensional mass cytometry. Arterioscler Thromb Vasc Biol 39:25–36
Mildner A, Marinkovic G, Jung S (2016) Murine monocytes: origins, subsets, fates, and functions. Microbiol Spectr 4. https://doi.org/10.1128/microbiolspec.MCHD-0033-2016
Narasimhan PB, Marcovecchio P, Hamers AAJ, Hedrick CC (2019) Nonclassical monocytes in health and disease. Annu Rev Immunol 37:439–456
Briseno CG, Haldar M, Kretzer NM, Wu X, Theisen DJ, Kc W, Durai V, Grajales-Reyes GE, Iwata A, Bagadia P et al (2016) Distinct transcriptional programs control cross-priming in classical and monocyte-derived dendritic cells. Cell Rep 15:2462–2474
Davies LC, Taylor PR (2015) Tissue-resident macrophages: then and now. Immunology 144:541–548
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, de Bruijn MF, Geissmann F, Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547–551
Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40:91–104
Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S (2019) Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20:29–39
Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, Zhang XM, Foo S, Nakamizo S, Duan K et al (2019) Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science (New York, NY):363. https://doi.org/10.1126/science.aau0964
Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD (2018) CCR2(+) monocyte-derived infiltrating macrophages are required for adverse cardiac remodeling during pressure overload. JACC Basic Transl Sci 3:230–244
Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL (2016) Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart (vol 111, pg 16029, 2014). Proc Natl Acad Sci U S A 113:E1414–E1414
Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A et al (2018) The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24:1234
Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SEW, Lavine KJ (2016) Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118:1498–U1127
Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao H-M, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ (2019) Tissue resident CCR2-and CCR2+cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124:263–278
Katz MG, Fargnoli AS, Gubara SM, Chepurko E, Bridges CR, Hajjar RJ (2019) Surgical and physiological challenges in the development of left and right heart failure in rat models. Heart Fail Rev 24:759–777
Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D (2019) Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol 114:19
Chen XH, Ruan CC, Ge Q, Ma Y, Xu JZ, Zhang ZB, Lin JR, Chen DR, Zhu DL, Gao PJ (2018) Deficiency of complement C3a and C5a receptors prevents angiotensin II-induced hypertension via regulatory T cells. Circ Res 122:970–983
Sharma RK, Yang T, Oliveira AC, Lobaton GO, Aquino V, Kim S, Richards EM, Pepine CJ, Sumners C, Raizada MK (2019) Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res 124:727–736
Byrne JA, Grieve DJ, Bendall JK, Li JM, Gove C, Lambeth JD, Cave AC, Shah AM (2003) Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res 93:802–805
Malhotra R, Sadoshima J, Brosius FC 3rd, Izumo S (1999) Mechanical stretch and angiotensin II differentially upregulate the renin-angiotensin system in cardiac myocytes in vitro. Circ Res 85:137–146
Ding Y, Wang Y, Jia Q, Wang X, Lu Y, Zhang A, Lv S, Zhang J (2020) Morphological and functional characteristics of animal models of myocardial fibrosis induced by pressure overload. Int J Hypertens 2020:3014693
Richards DA, Aronovitz MJ, Calamaras TD, Tam K, Martin GL, Liu P, Bowditch HK, Zhang P, Huggins GS, Blanton RM (2019) Distinct phenotypes induced by three degrees of transverse aortic constriction in mice. Sci Rep 9:5844
Wang L, Zhang YL, Lin QY, Liu Y, Guan XM, Ma XL, Cao HJ, Liu Y, Bai J, Xia YL, du J, Li HH (2018) CXCL1-CXCR2 axis mediates angiotensin II-induced cardiac hypertrophy and remodelling through regulation of monocyte infiltration. Eur Heart J 39:1818–1831
Luo YX, Tang X, An XZ, Xie XM, Chen XF, Zhao X, Hao DL, Chen HZ, Liu DP (2017) SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur Heart J 38:1389–1398
Zhang ZZ, Wang W, Jin HY, Chen X, Cheng YW, Xu YL, Song B, Penninger JM, Oudit GY, Zhong JC (2017) Apelin is a negative regulator of angiotensin II-mediated adverse myocardial remodeling and dysfunction. Hypertension (Dallas, Tex: 1979) 70:1165–1175
Patrucco E, Domes K, Sbroggió M, Blaich A, Schlossmann J, Desch M, Rybalkin SD, Beavo JA, Lukowski R, Hofmann F (2014) Roles of cGMP-dependent protein kinase I (cGKI) and PDE5 in the regulation of Ang II-induced cardiac hypertrophy and fibrosis. Proc Natl Acad Sci U S A 111:12925–12929
Bollen Y, Post J, Koo BK, Snippert HJG (2018) How to create state-of-the-art genetic model systems: strategies for optimal CRISPR-mediated genome editing. Nucleic Acids Res 46:6435–6454
Kim H, Kim M, Im SK, Fang S (2018) Mouse Cre-LoxP system: general principles to determine tissue-specific roles of target genes. Lab Anim Res 34:147–159
Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A (2018) Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol 233:6425–6440
Willeford A, Suetomi T, Nickle A, Hoffman HM, Miyamoto S, Heller Brown J (2018) CaMKIIdelta-mediated inflammatory gene expression and inflammasome activation in cardiomyocytes initiate inflammation and induce fibrosis. JCI Insight 3. https://doi.org/10.1172/jci.insight.97054
Suetomi T, Willeford A, Brand CS, Cho Y, Ross RS, Miyamoto S, Brown JH (2018) Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/calmodulin-dependent protein kinase II delta signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 138:2530–2544
Qi D, Wei M, Jiao S, Song Y, Wang X, Xie G, Taranto J, Liu Y, Duan Y, Yu B, Li H, Shah YM, Xu Q, du J, Gonzalez FJ, Qu A (2019) Hypoxia inducible factor 1alpha in vascular smooth muscle cells promotes angiotensin II-induced vascular remodeling via activation of CCL7-mediated macrophage recruitment. Cell Death Dis 10:544
Ikeda J, Ichiki T, Matsuura H, Inoue E, Kishimoto J, Watanabe A, Sankoda C, Kitamoto S, Tokunou T, Takeda K, Fong GH, Sunagawa K (2013) Deletion of Phd2 in myeloid lineage attenuates hypertensive cardiovascular remodeling. J Am Heart Assoc 2. https://doi.org/10.1161/JAHA.113.000178
Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K (2018) Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1beta/NLRP3 inflammasome. J Am Coll Cardiol 71:875–886
Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K (2018) CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 123:335–341
Wang Z, Xu Y, Wang M, Ye J, Liu J, Jiang H, Ye D, Wan J (2018) TRPA1 inhibition ameliorates pressure overload-induced cardiac hypertrophy and fibrosis in mice. EBioMedicine 36:54–62
Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, Fu C, Wynshaw-Boris A, Hu R, Schwartz MA, Fujioka H, Richardson B, Cameron MJ, Hayashi H, Stamler JS, Jain MK (2018) Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A 115:E4661–e4669
Glasenapp A, Derlin K, Wang Y, Bankstahl M, Meier M, Wollert KC, Bengel FM, Thackeray JT (2020) Multimodality imaging of inflammation and ventricular remodeling in pressure-overload heart failure. J Nuclear Med 61:590–596
Weisheit C, Zhang Y, Faron A, Koepke O, Weisheit G, Steinstraesser A, Frede S, Meyer R, Boehm O, Hoeft A et al (2014) Ly6C(low) and not Ly6C(high) macrophages accumulate first in the heart in a model of murine pressure-overload. PLoS One 9. https://doi.org/10.1371/journal.pone.0112710
Fujiu K, Shibata M, Nakayama Y, Ogata F, Matsumoto S, Noshita K, Iwami S, Nakae S, Komuro I, Nagai R, Manabe I (2017) A heart-brain-kidney network controls adaptation to cardiac stress through tissue macrophage activation. Nat Med 23:611–622
Patel B, Ismahil MA, Hamid T, Bansal SS, Prabhu SD (2017) Mononuclear phagocytes are dispensable for cardiac remodeling in established pressure-overload heart failure. PLoS One 12:e0170781
Miller MC, Mayo KH (2017) Chemokines from a structural perspective. Int J Mol Sci 18. https://doi.org/10.3390/ijms18102088
Stone MJ, Hayward JA, Huang C, Huma ZE, Sanchez J (2017) Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci 18. https://doi.org/10.3390/ijms18020342
Shen JZ, Morgan J, Tesch GH, Fuller PJ, Young MJ (2014) CCL2-dependent macrophage recruitment is critical for mineralocorticoid receptor-mediated cardiac fibrosis, inflammation, and blood pressure responses in male mice. Endocrinology 155:1057–1066
Nemska S, Monassier L, Gassmann M, Frossard N, Tavakoli R (2016) Kinetic mRNA profiling in a rat model of left-ventricular hypertrophy reveals early expression of chemokines and their receptors. PLoS One 11:e0161273
Collado A, Marques P, Escudero P, Rius C, Domingo E, Martinez-Hervas S, Real JT, Ascaso JF, Piqueras L, Sanz MJ (2018) Functional role of endothelial CXCL16/CXCR6-platelet-leucocyte axis in angiotensin II-associated metabolic disorders. Cardiovasc Res 114:1764–1775
Salvador AM, Nevers T, Velazquez F, Aronovitz M, Wang B, Abadia Molina A, Jaffe IZ, Karas RH, Blanton RM, Alcaide P (2016) Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc 5:e003126
Zhang YL, Geng C, Yang J, Fang J, Yan X, Li PB, Zou LX, Chen C, Guo SB, Li HH, Liu Y (2019) Chronic inhibition of chemokine receptor CXCR2 attenuates cardiac remodeling and dysfunction in spontaneously hypertensive rats. Biochim Biophys Acta Mol basis Dis 1865:165551
Lin QY, Lang PP, Zhang YL, Yang XL, Xia YL, Bai J, Li HH (2019) Pharmacological blockage of ICAM-1 improves angiotensin II-induced cardiac remodeling by inhibiting adhesion of LFA-1(+) monocytes. Am J Phys Heart Circ Phys 317:H1301–h1311
Xu X, Hua Y, Nair S, Bucala R, Ren J (2014) Macrophage migration inhibitory factor deletion exacerbates pressure overload-induced cardiac hypertrophy through mitigating autophagy. Hypertension (Dallas, Tex: 1979) 63:490–499
Koga K, Kenessey A, Ojamaa K (2013) Macrophage migration inhibitory factor antagonizes pressure overload-induced cardiac hypertrophy. Am J Phys Heart Circ Phys 304:H282–H293
Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM et al (2014) Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115:284–295
Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, Rosenthal NA (2012) An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7:e36814
DeBerge M, Zhang S, Glinton K, Grigoryeva L, Hussein I, Vorovich E, Ho K, Luo X, Thorp EB (2017) Efferocytosis and outside-in signaling by cardiac phagocytes. Links to repair, cellular programming, and intercellular crosstalk in heart. Front Immunol 8:1428
Gomez I, Duval V, Silvestre JS (2018) Cardiomyocytes and macrophages discourse on the method to govern cardiac repair. Front Cardiovasc Med 5:134
Hulsmans M, Clauss S, Xiao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G et al (2017) Macrophages facilitate electrical conduction in the heart. Cell 169:510–522.e520
Kimura A, Ishida Y, Furuta M, Nosaka M, Kuninaka Y, Taruya A, Mukaida N, Kondo T (2018) Protective roles of interferon-gamma in cardiac hypertrophy induced by sustained pressure overload. J Am Heart Assoc 7. https://doi.org/10.1161/jaha.117.008145
Tindemans I, Serafini N, Di Santo JP, Hendriks RW (2014) GATA-3 function in innate and adaptive immunity. Immunity 41:191–206
Yang M, Song L, Wang L, Yukht A, Ruther H, Li F, Qin M, Ghiasi H, Sharifi BG, Shah PK (2018) Deficiency of GATA3-positive macrophages improves cardiac function following myocardial infarction or pressure overload hypertrophy. J Am Coll Cardiol 72:885–904
Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR (2009) Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation 119:269–280
Bradshaw AD (2016) The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: does expression of SPARC by macrophages influence outcomes? J Mol Cell Cardiol 93:156–161
McDonald LT, Zile MR, Zhang Y, Van Laer AO, Baicu CF, Stroud RE, Jones JA, LaRue AC, Bradshaw AD (2018) Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis. Am J Phys Heart Circ Phys 315:H92–h100
Lu TX, Rothenberg ME (2018) MicroRNA. J Allergy Clin Immunol 141:1202–1207
Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stoger L, Wijnands E et al (2013) Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation 128:1420–1432
Lee T-M, Harn H-J, Chiou T-W, Chuang M-H, Chen C-H, Chuang C-H, Lin P-C, Lin S-Z (2019) Remote transplantation of human adipose-derived stem cells induces regression of cardiac hypertrophy by regulating the macrophage polarization in spontaneously hypertensive rats. Redox Biol 27. https://doi.org/10.1016/j.redox.2019.101170
Kallikourdis M, Martini E, Carullo P, Sardi C, Roselli G, Greco CM, Vignali D, Riva F, Ormbostad Berre AM, Stolen TO et al (2017) T cell costimulation blockade blunts pressure overload-induced heart failure. Nat Commun 8:14680
Zhao RR, Ackers-Johnson M, Stenzig J, Chen C, Ding T, Zhou Y, Wang P, Ng SL, Li PY, Teo G, Rudd PM, Fawcett JW, Foo RSY (2018) Targeting chondroitin sulfate glycosaminoglycans to treat cardiac fibrosis in pathological remodeling. Circulation 137:2497–2513
Bosch L, de Haan J, Seijkens T, van Tiel C, Brans M, Pasterkamp G, Lutgens E, de Jager S (2019) Small molecule-mediated inhibition of CD40-TRAF6 reduces adverse cardiac remodelling in pressure overload induced heart failure. Int J Cardiol 279:141–144
Zheng RH, Bai XJ, Zhang WW, Wang J, Bai F, Yan CP, James EA, Bose HS, Wang NP, Zhao ZQ (2019) Liraglutide attenuates cardiac remodeling and improves heart function after abdominal aortic constriction through blocking angiotensin II type 1 receptor in rats. Drug Des Dev Ther 13:2745–2757
Li R, Lu K, Wang Y, Chen M, Zhang F, Shen H, Yao D, Gong K, Zhang Z (2017) Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem Biophys Res Commun 485:69–75
Zhang X, Meng F, Song J, Zhang L, Wang J, Li D, Li L, Dong P, Yang B, Chen Y (2016) Pentoxifylline ameliorates cardiac fibrosis, pathological hypertrophy, and cardiac dysfunction in angiotensin II-induced hypertensive rats. J Cardiovasc Pharmacol 67:76–85
Hashimoto T, Sivakumaran V, Carnicer R, Zhu G, Hahn VS, Bedja D, Recalde A, Duglan D, Channon KM, Casadei B, Kass DA (2016) Tetrahydrobiopterin protects against hypertrophic heart disease independent of myocardial nitric oxide synthase coupling. J Am Heart Assoc 5:e003208
Podaru MN, Fields L, Kainuma S, Ichihara Y, Hussain M, Ito T, Kobayashi K, Mathur A, D'Acquisto F, Lewis-McDougall F et al (2019) Reparative macrophage transplantation for myocardial repair: a refinement of bone marrow mononuclear cell-based therapy. Basic Res Cardiol 114:34
Padmanabhan S, Joe B (2017) Towards precision medicine for hypertension: a review of genomic, epigenomic, and microbiomic effects on blood pressure in experimental rat models and humans. Physiol Rev 97:1469–1528
Huan T, Esko T, Peters MJ, Pilling LC, Schramm K, Schurmann C, Chen BH, Liu C, Joehanes R, Johnson AD, Yao C, Ying SX, Courchesne P, Milani L, Raghavachari N, Wang R, Liu P, Reinmaa E, Dehghan A, Hofman A, Uitterlinden AG, Hernandez DG, Bandinelli S, Singleton A, Melzer D, Metspalu A, Carstensen M, Grallert H, Herder C, Meitinger T, Peters A, Roden M, Waldenberger M, Dörr M, Felix SB, Zeller T, International Consortium for Blood Pressure GWAS (ICBP), Vasan R, O'Donnell CJ, Munson PJ, Yang X, Prokisch H, Völker U, van Meurs JBJ, Ferrucci L, Levy D (2015) A meta-analysis of gene expression signatures of blood pressure and hypertension. PLoS Genet 11:e1005035
Liu X, Meng F, Yang P (2013) Association study of CD36 single nucleotide polymorphisms with essential hypertension in the Northeastern Han Chinese. Gene 527:410–415
Ehret GB, O'Connor AA, Weder A, Cooper RS, Chakravarti A (2009) Follow-up of a major linkage peak on chromosome 1 reveals suggestive QTLs associated with essential hypertension: GenNet study. Eur J Hum Genet 17:1650–1657
Papalexi E, Satija R (2018) Single-cell RNA sequencing to explore immune cell heterogeneity. Nat Rev Immunol 18:35–45
See P, Lum J, Chen J, Ginhoux F (2018) A single-cell sequencing guide for immunologists. Front Immunol 9:2425
Funding
This work was supported by grants from the National Natural Science Foundation of China (81900219, 81530012, 81800216), the Fundamental Research Funds for the Central Universities (2042019kf0062, 2042018kf1032), Development Center for Medical Science and Technology National Health and Family Planning Commission of the People’s Republic of China (2016ZX-008-01), and National Key R&D Program of China (2018YFC1311300).
Author information
Authors and Affiliations
Contributions
Dan Yang, Han-Qing Liu, Di Fan, and Qi-Zhu Tang contributed to the conception and design of the review; Fang-Yuan Liu, Nan Tang, Zhen Guo, Shu-Qing Ma, Peng An, and Ming-Yu Wang performed the literature search and data analysis; the first draft of the manuscript was written by Dan Yan and Han-Qing Liu; Hai-Ming Wu, Zheng Yang, Di Fan, and Qi-Zhu Tang critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, D., Liu, HQ., Liu, FY. et al. Critical roles of macrophages in pressure overload-induced cardiac remodeling. J Mol Med 99, 33–46 (2021). https://doi.org/10.1007/s00109-020-02002-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-02002-w