Abstract
In patients with interstitial pneumonia, pulmonary fibrosis is an irreversible condition that can cause respiratory failure. Novel treatments for pulmonary fibrosis are necessary. Inflammation is thought to activate lung fibroblasts, resulting in pulmonary fibrosis. Of the known inflammatory molecules, we have focused on S100A8/A9 from the onset of inflammation to the subsequent progression of inflammation. Our findings confirmed the high expression of S100A8/A9 in specimens from patients with pulmonary fibrosis. An active role of S100A8/A9 was demonstrated not only in the proliferation of fibroblasts but also in the fibroblasts’ differentiation to myofibroblasts (the active form of fibroblasts). S100A8/A9 also forced fibroblasts to upregulate the production of collagen. These effects were induced via the receptor of S100A8/A9, i.e., the receptor for advanced glycation end products (RAGE), on fibroblasts. The anti-S100A8/A9 neutralizing antibody inhibited the effects of S100A8/A9 on fibroblasts and suppressed the progression of fibrosis in bleomycin (BLM)-induced pulmonary fibrosis mouse model. Our findings strongly suggest a crucial role of S100A8/A9 in pulmonary fibrosis and the usefulness of S100A8/A9-targeting therapy for fibrosis interstitial pneumonia.
Highlights
-
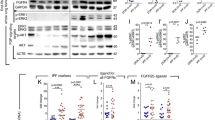
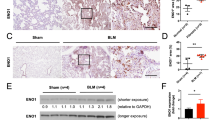
S100A8/A9 level is highly upregulated in the IPF patients’ lungs as well as the blood.
-
S100A8/A9 promotes not only the growth of fibroblasts but also differentiation to myofibroblasts.
-
The cell surface RAGE acts as a crucial receptor to the extracellular S100A8/A9 in fibroblasts.
-
The anti-S100A8/A9 antibody effectively suppresses the progression of IPF in a mouse model.
Graphical abstract

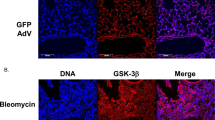
In idiopathic pulmonary fibrosis (IPF), S100A8/A9, a heterodimer composed of S100A8 and S100A9 proteins, plays a crucial role in the onset of inflammation and the subsequent formation of a feed-forward inflammatory loop that promotes fibrosis. (1) The local, pronounced increase in S100A8/A9 in the injured inflammatory lung region—which is provided mainly by the activated neutrophils and macrophages—exerts strong inflammatory signals accompanied by dozens of inflammatory soluble factors including cytokines, chemokines, and growth factors that further act to produce and secrete S100A8/A9, eventually making a sustainable inflammatory circuit that supplies an indefinite presence of S100A8/A9 in the extracellular space with a mal-increased level. (2) The elevated S100A8/A9 compels fibroblasts to activate through receptor for advanced glycation end products (RAGE), one of the major S100A8/A9 receptors, resulting in the activation of NFκB, leading to fibroblast mal-events (e.g., elevated cell proliferation and transdifferentiation to myofibroblasts) that actively produce not only inflammatory cytokines but also collagen matrices. (3) Finally, the S100A8/A9-derived activation of lung fibroblasts under a chronic inflammation state leads to fibrosis events and constantly worsens fibrosis in the lung. Taken together, these findings suggest that the extracellular S100A8/A9 heterodimer protein is a novel mainstay soluble factor for IPF that exerts many functions as described above (1–3). Against this background, we herein applied the developed S100A8/A9 neutralizing antibody to prevent IPF. The IPF imitating lung fibrosis in an IPF mouse model was effectively blocked by treatment with the antibody, leading to enhanced survival. The developed S100A8/A9 antibody, as an innovative novel biologic, may help shed light on the difficulties encountered with IPF therapy in clinical settings.





Similar content being viewed by others
Abbreviations
- ALCAM:
-
Activated leukocyte cell adhesion molecule
- α-SMA:
-
α-smooth muscle actin
- BALF:
-
Bronchoalveolar lavage fluid
- BLM:
-
Bleomycin
- ECM:
-
Extracellular matrix
- EMMPRIN:
-
Extracellular matrix metalloproteinase inducer
- EMSA:
-
Electrophoretic mobility shift assay
- GVHD:
-
Graft-versus-host disease
- H&E:
-
Hematoxylin and eosin
- HMGB1:
-
High-mobility group box1 protein
- IHC:
-
Immunohistochemistry
- IPF:
-
Idiopathic pulmonary fibrosis
- LAM:
-
Lymphangioleiomyomatosis
- MCAM:
-
Melanoma cell adhesion molecule
- NFκB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NPTN:
-
Neuroplastin
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PPH:
-
Primary pulmonary hypertension
- RAGE:
-
Receptor for advanced glycation end products
- SSSR:
-
S100 soil sensor receptor
References
Travis WD, Costabel U, Hansell DM, King TE, Jr., Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, Behr J, Bouros D, Brown KK, Colby TV, Collard HR, Cordeiro CR, Cottin V, Crestani B, Drent M, Dudden RF, Egan J, Flaherty K, Hogaboam C, Inoue Y, Johkoh T, Kim DS, Kitaichi M, Loyd J, Martinez FJ, Myers J, Protzko S, Raghu G, Richeldi L, Sverzellati N, Swigris J, Valeyre D, Pneumonias AECoII (2013) An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 188 (6):733-748.
Meyer KC, Decker CA (2017) Role of pirfenidone in the management of pulmonary fibrosis. Ther Clin Risk Manag 13:427–437
Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, Egan JJ, Lambrecht BN, Lories R, Parfrey H, Prasse A, Robalo-Cordeiro C, Verbeken E, Verschakelen JA, Wells AU, Verleden GM (2013) The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J 41(5):1207–1218
Chambers RC, Mercer PF (2015) Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc 12(Suppl 1):S16–S20
Barratt SL, Creamer A, Hayton C, Chaudhuri N (2018) Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med 7(8). https://doi.org/10.3390/jcm7080201
Nathan SD, Costabel U, Glaspole I, Glassberg MK, Lancaster LH, Lederer DJ, Pereira CA, Trzaskoma B, Morgenthien EA, Limb SL, Wells AU (2019) Efficacy of pirfenidone in the context of multiple disease progression events in patients with idiopathic pulmonary fibrosis. Chest 155(4):712–719
Heukels P, Moor CC, von der Thusen JH, Wijsenbeek MS, Kool M (2019) Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 147:79–91
Nakayama S, Mukae H, Sakamoto N, Kakugawa T, Yoshioka S, Soda H, Oku H, Urata Y, Kondo T, Kubota H, Nagata K, Kohno S (2008) Pirfenidone inhibits the expression of HSP47 in TGF-beta1-stimulated human lung fibroblasts. Life Sci 82(3-4):210–217
Shaw TJ, Rognoni E (2020) Dissecting fibroblast heterogeneity in health and fibrotic disease. Curr Rheumatol Rep 22(8):33
Bringardner BD, Baran CP, Eubank TD, Marsh CB (2008) The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal 10(2):287–301
Nakamura K, Sakaguchi M, Matsubara H, Akagi S, Sarashina T, Ejiri K, Akazawa K, Kondo M, Nakagawa K, Yoshida M, Miyoshi T, Ogo T, Oto T, Toyooka S, Higashimoto Y, Fukami K, Ito H (2018) Crucial role of RAGE in inappropriate increase of smooth muscle cells from patients with pulmonary arterial hypertension. PLoS One 13(9):e0203046. https://doi.org/10.1371/journal.pone.0203046
Bresnick AR, Weber DJ, Zimmer DB (2015) S100 proteins in cancer. Nat Rev Cancer 15(2):96–109
Chen H, Xu C, Jin Q, Liu Z (2014) S100 protein family in human cancer. Am J Cancer Res 4(2):89–115
Sato H, Sakaguchi M, Yamamoto H, Tomida S, Aoe K, Shien K, Yoshioka T, Namba K, Torigoe H, Soh J, Tsukuda K, Tao H, Okabe K, Miyoshi S, Pass HI, Toyooka S (2018) Therapeutic potential of targeting S100A11 in malignant pleural mesothelioma. Oncogenesis 7(1):11
Mitsui Y, Tomonobu N, Watanabe M, Kinoshita R, Sumardika IW, Youyi C, Murata H, Yamamoto KI, Sadahira T, Rodrigo AGH, Takamatsu H, Araki K, Yamauchi A, Yamamura M, Fujiwara H, Inoue Y, Futami J, Saito K, Iioka H, Kondo E, Nishibori M, Toyooka S, Yamamoto Y, Nasu Y, Sakaguchi M (2019) Upregulation of mobility in pancreatic cancer cells by secreted S100A11 through activation of surrounding fibroblasts. Oncol Res 27(8):945–956
Leclerc E, Vetter SW (2015) The role of S100 proteins and their receptor RAGE in pancreatic cancer. Biochim Biophys Acta 1852(12):2706–2711
Song JS, Kang CM, Park CK, Yoon HK, Lee SY, Ahn JH, Moon HS (2011) Inhibitory effect of receptor for advanced glycation end products (RAGE) on the TGF-beta-induced alveolar epithelial to mesenchymal transition. Exp Mol Med 43(9):517–524
Kim JH, Oh SH, Kim EJ, Park SJ, Hong SP, Cheon JH, Kim TI, Kim WH (2012) The role of myofibroblasts in upregulation of S100A8 and S100A9 and the differentiation of myeloid cells in the colorectal cancer microenvironment. Biochem Biophys Res Commun 423(1):60–66
Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ, Geczy CL (2013) Functions of S100 proteins. Curr Mol Med 13(1):24–57
Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J (2018) S100A8/A9 in Inflammation. Front Immunol 9:1298
Hibino T, Sakaguchi M, Miyamoto S, Yamamoto M, Motoyama A, Hosoi J, Shimokata T, Ito T, Tsuboi R, Huh NH (2013) S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res 73(1):172–183
Sakaguchi M, Yamamoto M, Miyai M, Maeda T, Hiruma J, Murata H, Kinoshita R, Winarsa Ruma IM, Putranto EW, Inoue Y, Morizane S, Huh NH, Tsuboi R, Hibino T (2016) Identification of an S100A8 receptor neuroplastin-beta and its heterodimer formation with EMMPRIN. J Invest Dermatol 136(11):2240–2250
Chen Y, Sumardika IW, Tomonobu N, Winarsa Ruma IM, Kinoshita R, Kondo E, Inoue Y, Sato H, Yamauchi A, Murata H, Yamamoto KI, Tomida S, Shien K, Yamamoto H, Soh J, Liu M, Futami J, Sasai K, Katayama H, Kubo M, Putranto EW, Hibino T, Sun B, Nishibori M, Toyooka S, Sakaguchi M (2019) Melanoma cell adhesion molecule is the driving force behind the dissemination of melanoma upon S100A8/A9 binding in the original skin lesion. Cancer Lett 452:178–190
Sumardika IW, Chen Y, Tomonobu N, Kinoshita R, Ruma IMW, Sato H, Kondo E, Inoue Y, Yamauchi A, Murata H, Yamamoto KI, Tomida S, Shien K, Yamamoto H, Soh J, Futami J, Putranto EW, Hibino T, Nishibori M, Toyooka S, Sakaguchi M (2019) Neuroplastin-beta mediates S100A8/A9-induced lung cancer disseminative progression. Mol Carcinog 58(6):980–995
Kinoshita R, Sato H, Yamauchi A, Takahashi Y, Inoue Y, Sumardika IW, Chen Y, Tomonobu N, Araki K, Shien K, Tomida S, Torigoe H, Namba K, Kurihara E, Ogoshi Y, Murata H, Yamamoto KI, Futami J, Putranto EW, Ruma IMW, Yamamoto H, Soh J, Hibino T, Nishibori M, Kondo E, Toyooka S, Sakaguchi M (2019) exSSSRs (extracellular S100 soil sensor receptors)-Fc fusion proteins work as prominent decoys to S100A8/A9-induced lung tropic cancer metastasis. Int J Cancer 144(12):3138–3145
Hiratsuka S, Ishibashi S, Tomita T, Watanabe A, Akashi-Takamura S, Murakami M, Kijima H, Miyake K, Aburatani H, Maru Y (2013) Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commun 4:1853
Kinoshita R, Sato H, Yamauchi A, Takahashi Y, Inoue Y, Sumardika IW, Chen Y, Tomonobu N, Araki K, Shien K, Tomida S, Torigoe H, Namba K, Kurihara E, Ogoshi Y, Murata H, Yamamoto KI, Futami J, Putranto EW, Ruma IMW, Yamamoto H, Soh J, Hibino T, Nishibori M, Kondo E, Toyooka S, Sakaguchi M (2019) Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int J Cancer 145(2):569–575
Bargagli E, Olivieri C, Cintorino M, Refini RM, Bianchi N, Prasse A, Rottoli P (2011) Calgranulin B (S100A9/MRP14): a key molecule in idiopathic pulmonary fibrosis? Inflammation 34(2):85–91
Hara A, Sakamoto N, Ishimatsu Y, Kakugawa T, Nakashima S, Hara S, Adachi M, Fujita H, Mukae H, Kohno S (2012) S100A9 in BALF is a candidate biomarker of idiopathic pulmonary fibrosis. Respir Med 106(4):571–580
Tardif MR, Chapeton-Montes JA, Posvandzic A, Page N, Gilbert C, Tessier PA (2015) Secretion of S100A8, S100A9, and S100A12 by neutrophils involves reactive oxygen species and potassium efflux. J Immunol Res 2015:296149–296116
Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A, Hibino T, Kataoka K, Huh NH (2011) TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One 6(8):e23132. https://doi.org/10.1371/journal.pone.0023132
Schuliga M (2015) NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 5(3):1266–1283
Tomonobu N, Kinoshita R, Sakaguchi M (2020) S100 soil sensor receptors and molecular targeting therapy against them in cancer metastasis. Transl Oncol 13(4):100753
Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, Xiang Z, Han X (2018) TNF-alpha-induced NF-kappaB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J Cell Physiol 233(3):2409–2419
Wang Q, Wang J, Wang J, Hong S, Han F, Chen J, Chen G (2017) HMGB1 induces lung fibroblast to myofibroblast differentiation through NFkappaBmediated TGFbeta1 release. Mol Med Rep 15(5):3062–3068
Takamatsu H, Yamamoto KI, Tomonobu N, Murata H, Inoue Y, Yamauchi A, Sumardika IW, Chen Y, Kinoshita R, Yamamura M, Fujiwara H, Mitsui Y, Araki K, Futami J, Saito K, Iioka H, Ruma IMW, Putranto EW, Nishibori M, Kondo E, Yamamoto Y, Toyooka S, Sakaguchi M (2019) Extracellular S100A11 plays a critical role in spread of the fibroblast population in pancreatic cancers. Oncol Res 27(6):713–727
Yin W, Han J, Zhang Z, Han Z, Wang S (2018) Aloperine protects mice against bleomycin-induced pulmonary fibrosis by attenuating fibroblast proliferation and differentiation. Sci Rep 8(1):6265
Mercer PF, Woodcock HV, Eley JD, Plate M, Sulikowski MG, Durrenberger PF, Franklin L, Nanthakumar CB, Man Y, Genovese F, McAnulty RJ, Yang S, Maher TM, Nicholson AG, Blanchard AD, Marshall RP, Lukey PT, Chambers RC (2016) Exploration of a potent PI3 kinase/mTOR inhibitor as a novel anti-fibrotic agent in IPF. Thorax 71(8):701–711
Liu G, Zhai H, Zhang T, Li S, Li N, Chen J, Gu M, Qin Z, Liu X (2019) New therapeutic strategies for IPF: based on the “phagocytosis-secretion-immunization” network regulation mechanism of pulmonary macrophages. Biomed Pharmacother 118:109230
Shabani F, Farasat A, Mahdavi M, Gheibi N (2018) Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm Res 67(10):801–812
Hamada N, Maeyama T, Kawaguchi T, Yoshimi M, Fukumoto J, Yamada M, Yamada S, Kuwano K, Nakanishi Y (2008) The role of high mobility group box1 in pulmonary fibrosis. Am J Respir Cell Mol Biol 39(4):440–447
Yamaguchi K, Iwamoto H, Sakamoto S, Horimasu Y, Masuda T, Miyamoto S, Nakashima T, Ohshimo S, Fujitaka K, Hamada H, Hattori N (2020) Serum high-mobility group box 1 is associated with the onset and severity of acute exacerbation of idiopathic pulmonary fibrosis. Respirology 25(3):275–280
Lee CC, Wang CN, Lee YL, Tsai YR, Liu JJ (2015) High mobility group box 1 induced human lung myofibroblasts differentiation and enhanced migration by activation of MMP-9. PLoS One 10(2):e0116393. https://doi.org/10.1371/journal.pone.0116393
Tesarova P, Kalousova M, Zima T, Tesar V (2016) HMGB1, S100 proteins and other RAGE ligands in cancer - markers, mediators and putative therapeutic targets. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 160(1):1–10
Wolf L, Herr C, Niederstrasser J, Beisswenger C, Bals R (2017) Receptor for advanced glycation endproducts (RAGE) maintains pulmonary structure and regulates the response to cigarette smoke. PLoS One 12(7):e0180092. https://doi.org/10.1371/journal.pone.0180092
Englert JM, Kliment CR, Ramsgaard L, Milutinovic PS, Crum L, Tobolewski JM, Oury TD (2011) Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis. Int J Clin Exp Pathol 4(3):241–254
Hasaneen NA, Cao J, Pulkoski-Gross A, Zucker S, Foda HD (2016) Extracellular matrix metalloproteinase inducer (EMMPRIN) promotes lung fibroblast proliferation, survival and differentiation to myofibroblasts. Respir Res 17:17
Kratzer A, Chu HW, Salys J, Moumen Z, Leberl M, Bowler R, Cool C, Zamora M, Taraseviciene-Stewart L (2013) Endothelial cell adhesion molecule CD146: implications for its role in the pathogenesis of COPD. J Pathol 230(4):388–398
Futami J, Atago Y, Azuma A, Putranto EW, Kinoshita R, Murata H, Sakaguchi M (2016) An efficient method for the preparation of preferentially heterodimerized recombinant S100A8/A9 coexpressed in Escherichia coli. Biochem Biophys Rep 6:94–100
Shien K, Toyooka S, Ichimura K, Soh J, Furukawa M, Maki Y, Muraoka T, Tanaka N, Ueno T, Asano H, Tsukuda K, Yamane M, Oto T, Kiura K, Miyoshi S (2012) Prognostic impact of cancer stem cell-related markers in non-small cell lung cancer patients treated with induction chemoradiotherapy. Lung Cancer 77(1):162–167
Acknowledgments
We thank Dr. Shin Morizane and Dr. Tomoko Miyake (Department of Dermatology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences) for the preparation of the psoriasis patients’ skin tissue sections used herein as a representative positive control for evaluating the S100A8/A9 staining. We also gratefully acknowledge Dr. Seiichi Tanida (Advanced Science, Technology and Management Research Institute of Kyoto) and Dr. Michiaki Mishima (Kyoto University) for their critical advice to this study.
Funding
This research was supported by grants from the Acceleration Transformative Research for Medical Innovation (ACT-M) in the Japan Agency for Medical Research and Development (AMED) (no. JP20im0210119) and the JSPS KAKENHI (no. 17H03577 to M.S.; no 19H03746 to S.T.) and by funds to M.S. from the Smoking Research Foundation, the Terumo Life Science Foundation, and the Takeda Science Foundation.
Author information
Authors and Affiliations
Contributions
K.A. established the methodology, designed and performed most of the experiments, and analyzed the data. R.K. prepared S100A8/A9 antibody and performed the ELISA and PCR experiments. N.T. and Y.G. assisted with most of the animal experiments and assisted K.A. in the data evaluation. S.T. contributed to the data analysis and its evaluation and validation. Y.T., S.S., K.S. K.S., H.Y. M. O., S.S., and K.I. contributed to the collection of the series of biomaterials. A.T. and N.M. contributed to the establishment of the animal model and assisted K.A. with some of the animal experiments. K.Y. and H.M. performed the primary cell culture and participated in the confirmation of some of the data of the in vitro cell assays. M.S. performed the immunoprecipitation, Western blot analysis, and EMSA, and M.N. assisted M.S.’s experiments. S.T and M.S. designed and supervised the project and wrote, reviewed, and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
A couple of studies using clinical specimens (tissues and blood samples) were approved by the Okayama Medical School and Hospital’s Research Ethics Committee. The approved numbers are as follows: # 1910-017 and # 1906-033. Informed consent was obtained from individual patients for the use of their materials. Experimental protocols required for the animal studies were approved by the Animal Experiment Committee at Okayama University. The approved numbers are as follows: # OKU-2018901 and # OKU-2019350.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Araki, K., Kinoshita, R., Tomonobu, N. et al. The heterodimer S100A8/A9 is a potent therapeutic target for idiopathic pulmonary fibrosis. J Mol Med 99, 131–145 (2021). https://doi.org/10.1007/s00109-020-02001-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-02001-x