Abstract
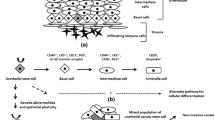
The expression of inducible nitric oxide (NO) synthase (iNOS) in human bladder cancer (BC) is a poor prognostic factor associated with invasion and tumor recurrence. Here, we evaluated the relevance of iNOS expression in BC progression and in cancer stem cell (CSC) maintenance in a murine BC model. Also, iNOS expression and CSC markers were analyzed in human BC samples. iNOS inhibitors (L-NAME or 1400W) or shRNA were used on murine BC model with different iNOS expressions and invasiveness grades: MB49 (iNOS+, non-muscle invasive (NMI)) and MB49-I (iNOS++, muscle invasive (MI)), in order to analyzed cell proliferation, tumor growth, angiogenesis, number of CSC, and pluripotential marker expression. iNOS, SOX2, Oct4, and Nanog expressions were also analyzed in human BC samples by qPCR and immunohistochemistry. iNOS inhibtion reduced parameters associated with tumor progression and reduced the number of CSC, wich resulted higher in MB49-I than in MB49, in concordance with the higher expression of SOX2, Oct4, and Nanog. The expression of SOX2 was notoriously diminished, when iNOS was inhibited only in the MI cell line. Similar results were observed in human samples, where MI tumors expressed higher levels of iNOS and pluripotential genes, in comparison to NMI tumors with a positive correlation between those and iNOS, suggesting that iNOS expression is associated with CSC. iNOS plays an important role in BC progression and CSC maintenance. Its inhibition could be a potential therapeutic target to eradicate CSC, responsible for tumor recurrences.
Key messages
• iNOS expression is involved in bladder tumor development, growth, and angiogenesis.
• iNOS expression is involved in bladder cancer stem cell generation and maintenance, playing an important role regulating their self-renewal capacity, especially in muscle invasive murine bladder cancer cells.
• iNOS expression is higher in human muscle invasive tumors, in association with a high expression of pluripotential genes, especially of SOX2.







Similar content being viewed by others
Data availability
Datasets are available from the corresponding author on reasonable request.
Abbreviations
- BC:
-
Bladder cancer
- NMI:
-
No muscle invasive
- LG:
-
Low grade
- HG:
-
High grade
- MI:
-
Muscle invasive
- TUR:
-
Transurethral resection
- CSC:
-
Cancer stem cell
- NO:
-
Nitric oxide
- iNOS:
-
Inducible nitric oxide synthase
- L-NAME:
-
Nω-Nitro-l-arginine methyl ester
- TBM:
-
Tumor-bearing mice
- SFE:
-
Sphere forming efficiency
References
Siegel R, Miller K, Jemal A (2015) Cancer statistics 2015. Cancer J Clin. https://doi.org/10.3322/caac.21254
Dickman KG, Fritsche HM, Grollman AP, Thalmann GN, Catto J (2015) Epidemiology and risk factors for upper urinary urothelial cancers, in: Up. Tract Urothelial Carcinoma:1–30. https://doi.org/10.1007/978-1-4939-1501-9_1
Mirza A, Choudhury A (2016) Bladder preservation for muscle invasive bladder cancer. Bl Cancer 2:151–163
Lytle NK, Barber AG, Reya T (2018) Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer. 18:669–680
Ma N, Thanan R, Kobayashi H, Hammam O, Wishahi M, El Leithy T, Hiraku Y, Amro EK, Oikawa S, Ohnishi S, Murata M, Kawanishi S (2011) Nitrative DNA damage and Oct3/4 expression in urinary bladder cancer with Schistosoma haematobium infection. Biochem. Biophys. Res. Commun. 414:344–349
Thanan R, Murata M, Ma N, Hammam O, Wishahi M, El Leithy T, Hiraku Y, Oikawa S, Kawanishi S (2012) Nuclear localization of COX-2 in relation to the expression of stemness markers in urinary bladder cancer. Mediators Inflamm. 2012:1–8
Chang CC, Shieh GS, Wu P, Lin CC, Shiau AL, Wu CL (2008) Oct-3/4 expression reflects tumor progression and regulates motility of bladder cancer cells. Cancer Res. 68:6281–6291
Zhang Y, Wang Z, Yu J, Shi J-Z, Wang C, Fu W-H, Chen Z-W, Yang J (2012) Cancer stem-like cells contribute to cisplatin resistance and progression in bladder cancer. Cancer Lett. https://doi.org/10.1016/j.canlet.2012.02.010
Ruan J, Wei B, Xu Z, Yang S, Zhou Y, Yu M, Liang J, Jin K, Huang X, Lu P, Cheng H (2013) Predictive value of Sox2 expression in transurethral resection specimens in patients with T1 bladder cancer. Med Oncol 30. https://doi.org/10.1007/s12032-012-0445-z
Yamanaka S, Takahashi K, Okita K, Nakagawa M (2007) Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2:3081–3089
Wamachi AN, Mayadev JS, Mungai PL, Magak PL, Ouma JH, Magambo JK, Muchiri EM, Koech DK, King CH, King CL (2004) Increased ratio of tumor necrosis factor-alpha to interleukin-10 production is associated with Schistosoma haematobium-induced urinary-tract morbidity. J. Infect. Dis. 190:2020–2030
Gakis G (2014) The role of inflammation in bladder cancer. In: Inflamm. CANCER, pp 183–196. https://doi.org/10.1007/978-3-0348-0837-8_8
Burke AJ, Sullivan FJ, Giles FJ, Glynn SA (2013) The yin and yang of nitric oxide in cancer progression. Carcinogenesis. https://doi.org/10.1093/carcin/bgt034
Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, Martin DN, Switzer CH, Hudson RS, Wink DA, Lee DH, Stephens RM, Ambs S (2010) Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest 120:3843–3854
Ambs S, Merriam WG, Bennett WP, Felley-Bosco E, Ogunfusika MO, Oser SM, Klein S, Shields PG, Billiar TR, Harris CC (1998) Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 58:334–341
Wang J, He P, Gaida M, Yang S, Schetter AJ, Gaedcke J, Ghadimi BM, Ried T, Yfantis H, Lee D, Weiss JM, Stauffer J, Hanna N, Alexander HR, Hussain SP (2016) Inducible nitric oxide synthase enhances disease aggressiveness in pancreatic cancer. Oncotarget 7:52993–53004
Okayama H, Saito M, Oue N, Weiss JM, Stauffer J, Takenoshita S, Wiltrout RH, Hussain SP, Harris CC (2013) NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression. Int. J. Cancer. 132:9–18
Brennan PA, Dennis S, Poller D, Quintero M, Puxeddu R, Thomas GJ (2008) Inducible nitric oxide synthase: correlation with extracapsular spread and enhancement of tumor cell invasion in head and neck squamous cell carcinoma. Head Neck. 30:208–214
Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, Lathia JD, Forrester MT, Lee J, Stamler JS, Goldman SA, Bredel M, McLendon RE, Sloan AE, Hjelmeland AB, Rich JN (2011) Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2. Cell. 146:53–66
Massi D, Franchi A, Sardi I, Magnelli L, Paglierani M, Borgognoni L, Reali UM, Santucci M (2001) Inducible nitric oxide synthase expression in benign and malignant cutaneous melanocytic lesions. J Pathol. 194:194–200
Sandes EO, Faletti AG, Riveros MD, Vidal Mdel C, Gimenez L, Casabe AR, Eijan AM (2005) Expression of inducible nitric oxide synthase in tumoral and non-tumoral epithelia from bladder cancer patients. Nitric Oxide 12:39–45 S1089-8603(04)00161-2 [pii]
Sandes EO, Lodillinsky C, Langle Y, Belgorosky D, Marino L, Gimenez L, Casabe AR, Eijan AM (2012) Inducible nitric oxide synthase and PPARgamma are involved in bladder cancer progression. J. Urol. 188:967–973
Li Y, Lin K, Yang Z, Han N, Quan X, Guo X, Li C (2017) Bladder cancer stem cells: clonal origin and therapeutic perspectives. Oncotarget. https://doi.org/10.18632/oncotarget.19112
Puglisi MA, Cenciarelli C, Tesori V, Cappellari M, Martini M, Di Francesco AM, Giorda E, Carsetti R, Ricci-Vitiani L, Gasbarrini A (2015) High nitric oxide production, secondary to inducible nitric oxide synthase expression, is essential for regulation of the tumour-initiating properties of colon cancer stem cells. J. Pathol. 236:479–490
Yongsanguanchai N, Pongrakhananon V, Mutirangura A, Rojanasakul Y, Chanvorachote P (2015) Nitric oxide induces cancer stem cell-like phenotypes in human lung cancer cells. Am. J. Physiol. - Cell Physiol. 308:C89–C100
Granados-Principal S, Liu Y, Guevara ML, Blanco E, Choi DS, Qian W, Patel T, Rodriguez AA, Cusimano J, Weiss HL, Zhao H, Landis MD, Dave B, Gross SS, Chang JC (2015) Inhibition of iNOS as a novel effective targeted therapy against triple-negative breast cancer. Breast Cancer Res. 17. https://doi.org/10.1186/s13058-015-0527-x
Lodillinsky C, Rodriguez V, Vauthay L, Sandes E, Casabé A, Eiján AM (2009) Novel invasive orthotopic bladder cancer model with high cathepsin B activity resembling human bladder cancer. J. Urol. 182:749–755
Belgorosky D, Langle Y, Cormick BPM, Colombo L, Sandes E, Eiján AM (2014) Inhibition of nitric oxide is a good therapeutic target for bladder tumors that express iNOS. Nitric Oxide - Biol. Chem. 36:11–18
Xu J, Xu X, Verstraete W (2000) Adaptation of E. Coli cell method for micro-scale nitrate measurement with the Griess reaction in culture media. J. Microbiol. Methods. 41:23–33
Sciacca M, Belgorosky D, Zambrano M, Gómez Escalante JI, Roca F, Langle YV, Sandes EO, Lodillinsky C, Eiján AM (2019) Inhibition of breast tumor growth by N(G)-nitro-L-arginine methyl ester (L-NAME) is accompanied by activation of fibroblasts. Nitric Oxide - Biol Chem 93. https://doi.org/10.1016/j.niox.2019.09.008
Langle YV, Belgorosky D, Prack McCormick B, Sahores A, Góngora A, Baldi A, Lanari C, Lamb C, Eiján AM (2016) FGFR3 Down-regulation is involved in Bacillus Calmette-Guérin induced bladder tumor growth inhibition. J. Urol. 195:188–197
Langle YV, Balarino NP, Belgorosky D, Cresta Morgado PD, Sandes EO, Marino L, Bilbao ER, Zambrano M, Lodillinsky C, Eiján AM (2020) Effect of nitric oxide inhibition in Bacillus Calmette-Guerin bladder cancer treatment. Nitric Oxide - Biol Chem. https://doi.org/10.1016/j.niox.2020.03.003
Cook T, Wang Z, Alber S, Liu K, Watkins SC, Vodovotz Y, Billiar TR, Blumberg D (2004) Nitric oxide and ionizing radiation synergistically promote apoptosis and growth inhibition of cancer by activating p53. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-04-2212
Fukumura D, Kashiwagi S, Jain RK (2006) The role of nitric oxide in tumour progression. Nat. Rev. Cancer. 6:521–534
Glynn SA (2019) Emerging novel mechanisms of action for nitric oxide in cancer progression. Curr Opin Physiol. https://doi.org/10.1016/j.cophys.2019.03.010
Batlle E, Clevers H (2017) Cancer stem cells revisited. Nat. Med. 23:1124–1134
Papaevangelou E, Whitley GS, Johnstone AP, Robinson SP, Howe FA (2016) Investigating the role of tumour cell derived iNOS on tumour growth and vasculature in vivo using a tetracycline regulated expression system. Int. J. Cancer. 138:2678–2687
Zhang S (2014) Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. https://doi.org/10.4252/wjsc.v6.i3.305
K. Weina, J. Utikal, SOX2 and cancer: current research and its implications in the clinic, Clin Transl Med. (2014). https://doi.org/10.1186/2001-1326-3-19, SOX2 and cancer: current research and its implications in the clinic.
Zhu F, Qian W, Zhang H, Liang Y, Wu M, Zhang Y, Zhang X, Gao Q, Li Y (2017) SOX2 is a marker for stem-like tumor cells in bladder cancer. Stem Cell Rep. https://doi.org/10.1016/j.stemcr.2017.07.004
Novak D, Hüser L, Elton JJ, Umansky V, Altevogt P, Utikal J (2019) SOX2 in development and cancer biology. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2019.08.007
Palumbo P, Miconi G, Cinque B, Lombardi F, La Torre C, Dehcordi SR, Galzio R, Cimini A, Giordano A, Cifone MG (2017) NOS2 expression in glioma cell lines and glioma primary cell cultures: correlation with neurosphere generation and SOX-2 expression. Oncotarget. https://doi.org/10.18632/oncotarget.16106
Yang N, Hui L, Wang Y, Yang H, Jiang X (2014) SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol. Rep. 31:2651–2659
Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, Markowitz D, Reisfeld RA, Luo Y (2013) Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine egfr/stat3/sox-2 signaling pathway. Stem Cells. https://doi.org/10.1002/stem.1281
Girouard J, Belgorosky D, Hamelin-Morrissette J, Boulanger V, D’Orio E, Ramla D, Perron R, Charpentier L, Van Themsche C, Eiján AM, Bérubé G, Reyes-Moreno C (2020) Molecular therapy with derivatives of amino benzoic acid inhibits tumor growth and metastasis in murine models of bladder cancer through inhibition of TNFα/NFΚB and iNOS/NO pathways. Biochem. Pharmacol. 176:113778
Hamelin-Morrissette J, Cloutier S, Girouard J, Belgorosky D, Eiján AM, Legault J, Reyes-Moreno C, Bérubé G (2015) Identification of an anti-inflammatory derivative with anti-cancer potential: the impact of each of its structural components on inflammatory responses in macrophages and bladder cancer cells. Eur J Med Chem 96. https://doi.org/10.1016/j.ejmech.2015.04.026
Fujita M, Somasundaram V, Basudhar D, Cheng RYS, Ridnour LA, Higuchi H, Imadome K, No JH, Bharadwaj G, Wink DA (2019) Role of nitric oxide in pancreatic cancer cells exhibiting the invasive phenotype. Redox Biol. https://doi.org/10.1016/j.redox.2019.101158
Hoskin PJ (n.d.) Clical trials.gov, 2015. https://clinicaltrials.gov/ct2/show/NCT01324115?term=nitric+oxide+inhibitor&cond=cancer&draw=2&rank=2.
Darcourt J (n.d.) Clinicaltrials.gov, 2018. https://clinicaltrials.gov/ct2/show/NCT03236935?term=nitric+oxide+inhibitor&cond=cancer&draw=2&rank=3.
Alameda F, Velarde JM, Carrato C, Vidal N, Arumí M, Naranjo D, Martinez-García M, Ribalta T, Balañá C (2019) Prognostic value of stem cell markers in glioblastoma. J Biomarkers 24:677–683
Maiuthed A, Bhummaphan N, Luanpitpong S, Mutirangura A, Aporntewan C, Meeprasert A, Rungrotmongkol T, Rojanasakul Y, Chanvorachote P (2018) Nitric oxide promotes cancer cell dedifferentiation by disrupting an Oct4:caveolin-1 complex: a new regulatory mechanism for cancer stem cell formation. J. Biol. Chem. 293:13534–13552
Wang R, Li Y, Tsung A, Huang H, Du Q, Yang M, Deng M, Xiong S, Wang X, Zhang L, Geller DA, Cheng B, Billiar TR (2018) INOS promotes CD24+CD133+ liver cancer stem cell phenotype through a TACE/ADAM17-dependent Notch signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 115:E10127–E10136
Acknowledgments
We are grateful to Dr. Carlos Spector, Maria Pilar Jerabek, Martina Rodriguez Olivera, and Lourdes Otamendi from UCES for their support. The authors thank Dr. Lionel Berthoux for his advice and technical assistance on shRNA iNOS knockdown. We are grateful also to the Emerging Leaders in The Americas Program (ELAP) scholarships of Canada. Also, we thank the animal facility from IOAHR and UQTR for their technical support. We thank Lic. Inés Kletzky for her critical English revision.
Funding
This study was supported by funding from IOAHR, Universidad de Buenos Aires, CONICET (PIP9671/14), UBACyT 20720150100001BA, Agencia de promoción Cientifica y Tecnologica - PICT 2017-0776, Cancer Institute of Canadian Institutes of Health Research (CIHR, number 392334), Cancer Research Society (CRS, number 22471), Fundación hermanos Agustín y Enrique Rocca, Escuela Técnica ORT, and Universidad de Ciencias Empresariales y Sociales (UCES).
Author information
Authors and Affiliations
Contributions
D. B. has performed the experimental design and carried out the experimental work, the interpretation of the results, drafting of the manuscript, and the discussion of the intellectual content. J. G. and J. H-M. had provided experimental ideas and had participated in silencing iNOS and in vivo imaging experiments. Y. V. L. has also provided experimental ideas, performed some of the qPCR experiments, and helped with the interpretation of the results; and also helped with in vivo assays and contributed in revising the draft critically for important intellectual content. L. M. contributed in ICH experiments. E. I. A. has participated with some qPCR experiments and contributed in revising the draft critically. H. M. provided human samples. Reyes-Moreno has contributed in conceptualization, methodology, resources, review and editing, supervision, and funding acquisition. A. M. E. has contributed in experimental design, funding acquisition, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Studies using patient samples were approved by the Institutional Ethics Committee from IOAHR according to the Declaration of Helsinki (CEI: 6/6/2013), with the corresponding informed consents from each patient. Mice were obtained from the animal facility of the IOAHR or Charles River Laboratories (Montréal, Canada) and handled in accordance with the ARRIVE guidelines. Protocols were approved by the Institutional Review Board of IOAHR (2012/02) and the animal care and use committee of the UQTR, Canada (2015-CRM-3).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Belgorosky, D., Girouard, J., Langle, Y.V. et al. Relevance of iNOS expression in tumor growth and maintenance of cancer stem cells in a bladder cancer model. J Mol Med 98, 1615–1627 (2020). https://doi.org/10.1007/s00109-020-01973-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01973-0