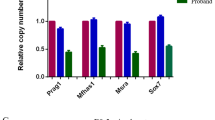
Abstract
Conotruncal heart defects (CTDs) are closely related to defective outflow tract (OFT) development, in which cardiac neural crest cells (CNCCs) play an indispensable role. However, the genetic etiology of CTDs remains unclear. Mesoderm posterior 2 (MESP2) is an important transcription factor regulating early cardiogenesis. Nevertheless, MESP2 variants have not been reported in congenital heart defect (CHD) patients. We first identified four MESP2 variants in 601 sporadic nonsyndromic CTD patients that were not detected in 400 healthy controls using targeted sequencing. Reverse transcription-quantitative PCR (RT-qPCR), immunohistochemistry, and immunofluorescence assays revealed MESP2 expression in the OFT of Carnegie stage (CS) 11, CS13, and CS15 human embryos and embryonic day (E) 8.5, E10, and E11.5 mouse embryos. Functional analyses in HEK 293T cells, HL-1 cells, JoMa1 cells, and primary mouse CNCCs revealed that MESP2 directly regulates the transcriptional activities of downstream CTD-related genes and promotes CNCC proliferation by regulating cell cycle factors. Three MESP2 variants, c.346G>C (p.G116R), c.921C>G (p.Y307X), and c.59A>T (p.Q20L), altered the transcriptional activities of MYOCD, GATA4, NKX2.5, and CFC1 and inhibited CNCC proliferation by upregulating p21cip1 or downregulating Cdk4. Based on our findings, MESP2 variants disrupted MESP2 function by interfering with CNCC proliferation during OFT development, which may contribute to CTDs.
Key messages
-
This study first analyzed MESP2 variants identified in sporadic nonsyndromic CTD patients.
-
MESP2 is expressed in the OFT of different stages of human and mouse embryos.
-
MESP2 regulates the transcriptional activities of downstream CTD-related genes and promotes CNCC proliferation by regulating cell cycle factor p21cip1 or Cdk4.






Similar content being viewed by others
References
Hoffman JI, Kaplan S, Liberthson RR (2004) Prevalence of congenital heart disease. Am Heart J 147(3):425–439
Hoffman J (2013) The global burden of congenital heart disease. Cardiovasc J Afr 24(4):141–145
van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, Roos-Hesselink JW (2011) Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 58(21):2241–2247
Boudjemline Y, Fermont L, Le Bidois J, Lyonnet S, Sidi D, Bonnet D (2001) Prevalence of 22q11 deletion in fetuses with conotruncal cardiac defects: a 6-year prospective study. J Pediatr 138(4):520–524
Tennstedt C, Chaoui R, Korner H, Dietel M (1999) Spectrum of congenital heart defects and extracardiac malformations associated with chromosomal abnormalities: results of a seven year necropsy study. Heart 82(1):34–39
Saga Y, Hata N, Koseki H, Taketo MM (1997) Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev 11(14):1827–1839
Liang Q, Xu C, Chen X, Li X, Lu C, Zhou P, Yin L, Qian R, Chen S, Ling Z et al (2015) The roles of Mesp family proteins: functional diversity and redundancy in differentiation of pluripotent stem cells and mammalian mesodermal development. Protein Cell 6(8):553–561
Kitajima S, Takagi A, Inoue T, Saga Y (2000) MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 127(15):3215–3226
Chiapparo G, Lin X, Lescroart F, Chabab S, Paulissen C, Pitisci L, Bondue A, Blanpain C (2016) Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration. J Cell Biol 213(4):463–477
Lahm H, Deutsch MA, Dressen M, Doppler S, Werner A, Horer J, Cleuziou J, Schreiber C, Bohm J, Laugwitz KL et al (2013) Mutational analysis of the human MESP1 gene in patients with congenital heart disease reveals a highly variable sequence in exon 1. Eur J Med Genet 56(11):591–598
Werner P, Latney B, Deardorff MA, Goldmuntz E (2016) MESP1 mutations in patients with congenital heart defects. Hum Mutat 37(3):308–314
Zhang M, Li FX, Liu XY, Huang RT, Xue S, Yang XX, Li YJ, Liu H, Shi HY, Pan X et al (2017) MESP1 lossoffunction mutation contributes to double outlet right ventricle. Mol Med Rep 16(3):2747–2754
Cornier AS, Staehling-Hampton K, Delventhal KM, Saga Y, Caubet JF, Sasaki N, Ellard S, Young E, Ramirez N, Carlo SE et al (2008) Mutations in the MESP2 gene cause spondylothoracic dysostosis/Jarcho-Levin syndrome. Am J Hum Genet 82(6):1334–1341
Keyte A, Hutson MR (2012) The neural crest in cardiac congenital anomalies. Different Res Biol Divers 84(1):25–40
Hutson MR, Kirby ML (2003) Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today 69(1):2–13
Waldo KL, Hutson MR, Stadt HA, Zdanowicz M, Zdanowicz J, Kirby ML (2005) Cardiac neural crest is necessary for normal addition of the myocardium to the arterial pole from the secondary heart field. Dev Biol 281(1):66–77
Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML (2005) Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol 281(1):78–90
Hutson MR, Kirby ML (2007) Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol 18(1):101–110
Kirby ML, Gale TF, Stewart DE (1983) Neural crest cells contribute to normal aorticopulmonary septation. Science 220(4601):1059–1061
Conway SJ, Bundy J, Chen J, Dickman E, Rogers R, Will BM (2000) Decreased neural crest stem cell expansion is responsible for the conotruncal heart defects within the splotch (Sp(2H))/Pax3 mouse mutant. Cardiovasc Res 47(2):314–328
Lo CW, Cohen MF, Huang GY, Lazatin BO, Patel N, Sullivan R, Pauken C, Park SM (1997) Cx43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev Genet 20(2):119–132
Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, Chin AJ, Park SM, Lazatin BO, Villabon S et al (1997) Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development (Camb, Engl) 124(7):1281–1292
Zhang X, Xu Y, Liu D, Geng J, Chen S, Jiang Z, Fu Q, Sun K (2015) A modified multiplex ligation-dependent probe amplification method for the detection of 22q11.2 copy number variations in patients with congenital heart disease. BMC Genomics 16(1):1–11
Etchevers H (2011) Primary culture of chick, mouse or human neural crest cells. Nat Protoc 6(10):1568–1577
Huang GY, Cooper ES, Waldo K, Kirby ML, Gilula NB, Lo CW (1998) Gap junction-mediated cell-cell communication modulates mouse neural crest migration. J Cell Biol 143(6):1725–1734
Morimoto M, Kiso M, Sasaki N, Saga Y (2006) Cooperative Mesp activity is required for normal somitogenesis along the anterior-posterior axis. Dev Biol 300(2):687–698
Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, Kyba M, Blanpain C (2008) Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell 3(1):69–84
David R, Brenner C, Stieber J, Schwarz F, Brunner S, Vollmer M, Mentele E, Muller-Hocker J, Kitajima S, Lickert H et al (2008) MesP1 drives vertebrate cardiovascular differentiation through Dkk-1-mediated blockade of Wnt-signalling. Nat Cell Biol 10(3):338–345
Haraguchi S, Kitajima S, Takagi A, Takeda H, Inoue T, Saga Y (2001) Transcriptional regulation of Mesp1 and Mesp2 genes: differential usage of enhancers during development. Mech Dev 108(1-2):59–69
Morimoto M, Takahashi Y, Endo M, Saga Y (2005) The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 435(7040):354–359
Pilon N, Raiwet D, Viger RS, Silversides DW (2008) Novel pre- and post-gastrulation expression of Gata4 within cells of the inner cell mass and migratory neural crest cells. Dev Dyn 237(4):1133–1143
Huang J, Cheng L, Li J, Chen M, Zhou D, Lu MM, Proweller A, Epstein JA, Parmacek MS (2008) Myocardin regulates expression of contractile genes in smooth muscle cells and is required for closure of the ductus arteriosus in mice. J Clin Invest 118(2):515–525
Ikle JM, Tavares AL, King M, Ding H, Colombo S, Firulli BA, Firulli AB, Targoff KL, Yelon D, Clouthier DE (2017) Nkx2.5 regulates endothelin converting enzyme-1 during pharyngeal arch patterning. Genesis (New York, NY: 2000) 55(3). https://doi.org/10.1002/dvg.23021
Obler D, Juraszek AL, Smoot LB, Natowicz MR (2008) Double outlet right ventricle: aetiologies and associations. J Med Genet 45(8):481–497
Zweier C, Sticht H, Aydin-Yaylagul I, Campbell CE, Rauch A (2007) Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet 80(3):510–517
Postma AV, van de Meerakker JB, Mathijssen IB, Barnett P, Christoffels VM, Ilgun A, Lam J, Wilde AA, Lekanne Deprez RH, Moorman AF (2008) A gain-of-function TBX5 mutation is associated with atypical Holt-Oram syndrome and paroxysmal atrial fibrillation. Circ Res 102(11):1433–1442
Posch MG, Gramlich M, Sunde M, Schmitt KR, Lee SH, Richter S, Kersten A, Perrot A, Panek AN, Al Khatib IH et al (2010) A gain-of-function TBX20 mutation causes congenital atrial septal defects, patent foramen ovale and cardiac valve defects. J Med Genet 47(4):230–235
Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z et al (2004) Mouse brain organization revealed through direct genome-scale TF expression analysis. Science 306(5705):2255–2257
Singh MK, Li Y, Li S, Cobb RM, Zhou D, Lu MM, Epstein JA, Morrisey EE, Gruber PJ (2010) Gata4 and Gata5 cooperatively regulate cardiac myocyte proliferation in mice. J Biol Chem 285(3):1765–1772
Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL (2008) GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol 28(17):5420–5431
Kasahara H, Ueyama T, Wakimoto H, Liu MK, Maguire CT, Converso KL, Kang PM, Manning WJ, Lawitts J, Paul DL et al (2003) Nkx2.5 homeoprotein regulates expression of gap junction protein connexin 43 and sarcomere organization in postnatal cardiomyocytes. J Mol Cell Cardiol 35(3):243–256
Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML (2001) Conotruncal myocardium arises from a secondary heart field. Development (Camb, Engl) 128(16):3179–3188
Li S, Wang DZ, Wang Z, Richardson JA, Olson EN (2003) The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc Natl Acad Sci U S A 100(16):9366–9370
Buckingham M, Meilhac S, Zaffran S (2005) Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 6(11):826–835
Shen MM, Schier AF (2000) The EGF-CFC gene family in vertebrate development. Trends Genet: TIG 16(7):303–309
Acknowledgments
We thank all the patients and control individuals who participated in the study. We thank Dr. Shubao Chen for providing helpful advice during the preparation of the manuscript. We thank the members of the Medical Laboratory and Department of Pediatric Cardiology, Shanghai Children’s Medical Center, for their indispensable assistance with peripheral blood collection. We thank the members of the Lab of Pediatric Cardiovascular and Department of Pediatric Cardiovascular, Xinhua Hospital, for providing technical assistance. We also thank the members of the Department of Obstetrics and Gynecology, Xinhua Hospital, for their collaborative assistance with human embryo collection.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant number 81670285, 81974021, and 81974012), the Major International (Regional) Joint Research Project of the National Natural Science Foundation of China (grant number 81720108003), the Shanghai Hospital Development Center (grant number SHDC12015102), and the Shanghai Municipal Health Bureau (grant number ZHYY-ZXYJHZX-1-04).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The human study was approved by the Ethics Committee of Xinhua Hospital (XHEC-2016-C-2016-303) and Shanghai Children’s Medical Center (SCMC-201015) and performed in accordance with the Declaration of Helsinki. Peripheral blood and embryos were collected from the participants after informed consent was obtained. The animal experiments were approved by the Ethics Committee of Xinhua Hospital (XHEC-2016-F-2016-197). All animal procedures conformed to the NIH Guidelines for the Care and Use of Laboratory Animals.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 4.38 mb)
Rights and permissions
About this article
Cite this article
Zhang, E., Yang, J., Liu, Y. et al. MESP2 variants contribute to conotruncal heart defects by inhibiting cardiac neural crest cell proliferation. J Mol Med 98, 1035–1048 (2020). https://doi.org/10.1007/s00109-020-01929-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01929-4