Abstract
Extracellular vesicles (EVs) have been growingly recognized as biomarkers and mediators of alcoholic liver disease (ALD) in human and mice. Here we characterized hepatocyte-derived EVs (HC-EVs) and their cargo for their biological functions in a novel murine model that closely resembles liver pathology observed in patients with alcoholic hepatitis (AH), the most severe spectrum of ALD. The numbers of circulating EVs and HC-EVs were significantly increased by 10-fold in AH mice compared with control mice. The miRNA (miR)–seq analysis detected 20 upregulated and 4 downregulated miRNAs (P < 0.001–0.05) in AH-HC-EVs. Treatment of murine primary hepatic stellate cells (HSCs) with AH-HC-EVs induced α-SMA (P < 0.05) and Col1a1 (P < 0.001). Smad7 and Nr1d2 genes, which were downregulated in HSCs from the AH mice, were predicted targets of 20 miRs upregulated in AH-HC-EVs. Among them were miR-27a and miR-181 which upon transfection in HSCs, indeed repressed Nr1d2, the quiescent HSC marker. AH-HC-EVs were also enriched with organelle proteins and mitochondrial DNA (10-fold, P < 0.05) and upregulated IL-1β and IL-17 production by hepatic macrophages (HMs) from AH mice in a TLR9-dependent manner. These results demonstrate HC-EV release is intensified in AH and suggest that AH-HC-EVs orchestrate liver fibrogenesis by directly targeting the quiescent HSC transcripts via a unique set of miRNAs and by amplifying HSC activation via DAMP-based induction of profibrogenic IL-1β and IL-17 by HMs.
Key messages
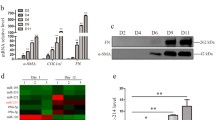
• Circulating EVs and HC-EVs were increased in AH mice compared with control mice
• AH-HC-EVs were enriched in miRNAs, organelle proteins, and mitochondrial DNA
• AH-HC-EVs increased cytokine production by AH-HMs in a TLR9-dependent manner





Similar content being viewed by others
Abbreviations
- ALD:
-
Alcoholic liver disease
- ASH:
-
Alcoholic steatohepatitis
- HCC:
-
Hepatocellular carcinoma
- EV:
-
Extracellular vesicle
- HC:
-
Hepatocyte
- HM:
-
Hepatic macrophage
- HC-EV:
-
Hepatocyte-derived EV
- HM-EV:
-
Hepatic macrophages-derived EV
- miRNA:
-
MicroRNA
References
Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG (1999) Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117:942–952
Mathurin P (2009) Alcohol and the liver. Gastroenterologie clinique et biologique 33:840–849
Nanji AA, Su GL, Laposata M, French SW (2002) Pathogenesis of alcoholic liver disease--recent advances. Alcohol Clin Exp Res 26:731–736
Feldstein AE, Gores GJ (2005) Apoptosis in alcoholic and nonalcoholic steatohepatitis. Frontiers in bioscience : a journal and virtual library 10:3093–3099
Thurman RG, Bradford BU, Iimuro Y, Knecht KT, Connor HD, Adachi Y, Wall C, Arteel GE, Raleigh JA, Forman DT, Mason RP (1997) Role of Kupffer cells, endotoxin and free radicals in hepatotoxicity due to prolonged alcohol consumption: studies in female and male rats. J Nutr 127:903S–906S
Nanji AA (2002) Role of Kupffer cells in alcoholic hepatitis. Alcohol 27:13–15
Rose ML, Rusyn I, Bojes HK, Belyea J, Cattley RC, Thurman RG (2000) Role of Kupffer cells and oxidants in signaling peroxisome proliferator-induced hepatocyte proliferation. Mutat Res 448:179–192
Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, Ho SB, Starkel P, Schnabl B, Ohno-Machado L et al (2017) Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology 65:475–490
Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, Xu J, Machida K, Tsukamoto H (2015) Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology 61:129–140
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J et al (2015) Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 4:27066
Gao B, Ahmad MF, Nagy LE, Tsukamoto H (2019) Inflammatory pathways in alcoholic steatohepatitis. J Hepatol 70:249–259
Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, Llorente C, Pan SQ, Yang Q, Li Y, Lazaro R, Ansong C, Smith RD, Bataller R, Morgan T, Schnabl B, Tsukamoto H (2018) Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 67:1737–1753
Lai KKY, Kweon SM, Chi F, Hwang E, Kabe Y, Higashiyama R, Qin L, Yan R, Wu RP, Lai K, Fujii N, French S, Xu J, Wang JY, Murali R, Mishra L, Lee JS, Ntambi JM, Tsukamoto H (2017) Stearoyl-CoA desaturase promotes liver fibrosis and tumor development in mice via a Wnt positive-signaling loop by stabilization of low-density lipoprotein-receptor-related proteins 5 and 6. Gastroenterology 152:1477–1491
Xu J, Chi F, Guo T, Punj V, Lee WN, French SW, Tsukamoto H (2015) NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest 125:1579–1590
Anders S, Huber W (2010) Differential expression analysis for sequence count data. Genome Biol 11:R106
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57:289–300
Kim J, Levy E, Ferbrache A, Stepanowsky P, Farcas C, Wang S, Brunner S, Bath T, Wu Y, Ohno-Machado L (2014) MAGI: a Node.js web service for fast microRNA-Seq analysis in a GPU infrastructure. Bioinformatics 30:2826–2827
Vlachos IS, Zagganas K, Paraskevopoulou MD, Georgakilas G, Karagkouni D, Vergoulis T, Dalamagas T, Hatzigeorgiou AG (2015) DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 43:W460–W466
Wang H, Qian WJ, Mottaz HM, Clauss TR, Anderson DJ, Moore RJ, Camp DG 2nd, Khan AH, Sforza DM, Pallavicini M et al (2005) Development and evaluation of a micro- and nanoscale proteomic sample preparation method. J Proteome Res 4:2397–2403
Yang J, Yin L, Lessner FH, Nakayasu ES, Payne SH, Fixen KR, Gallagher L, Harwood CS (2017) Genes essential for phototrophic growth by a purple alphaproteobacterium. Environ Microbiol 19:3567–3578
Bantscheff M, Schirle M, Sweetman G, Rick J, Kuster B (2007) Quantitative mass spectrometry in proteomics: a critical review. Anal Bioanal Chem 389:1017–1031
Schulze WX, Usadel B (2010) Quantitation in mass-spectrometry-based proteomics. Annu Rev Plant Biol 61:491–516
Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, Coffman RL, Candia A, Mehal WZ (2016) Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest 126:859–864
Tkach M, Thery C (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164:1226–1232
Liu X, Rosenthal SB, Meshgin N, Baglieri J, Musallam SG, Diggle K, Lam K, Wu R, Pan SQ, Chen Y, Dorko K, Presnell S, Benner C, Hosseini M, Tsukamoto H, Brenner D, Kisseleva T (2020) Primary alcohol-activated human and mouse hepatic stellate cells share similarities in gene-expression profiles. Hepatology communications 4:606–626
Liu X, Xu J, Brenner DA, Kisseleva T (2013) Reversibility of liver fibrosis and inactivation of fibrogenic myofibroblasts. Curr Pathobiol Rep 1:209–214
Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H (2016) Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology 64:2219–2233
Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo HL, Pinatel EM, Alisi A, Nobili V, Feldstein AE (2015) Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cellular and molecular gastroenterology and hepatology 1:646–663 e644
Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M, Österreicher CH, Stickel F, Ley K, Brenner DA, Kisseleva T (2012) Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology 143:765–776 e763
Fabre T, Kared H, Friedman SL, Shoukry NH (2014) IL-17A enhances the expression of profibrotic genes through upregulation of the TGF-beta receptor on hepatic stellate cells in a JNK-dependent manner. J Immunol 193:3925–3933
Bhan U, Ballinger MN, Zeng X, Newstead MJ, Cornicelli MD, Standiford TJ (2010) Cooperative interactions between TLR4 and TLR9 regulate interleukin 23 and 17 production in a murine model of gram negative bacterial pneumonia. PLoS One 5:e9896
Salloum N, Hussein HM, Jammaz R, Jiche S, Uthman IW, Abdelnoor AM, Rahal EA (2018) Epstein-Barr virus DNA modulates regulatory T-cell programming in addition to enhancing interleukin-17A production via Toll-like receptor 9. PLoS One 13:e0200546
Mansouri A, Gattolliat CH, Asselah T (2018) Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155:629–647
Hoek JB, Cahill A, Pastorino JG (2002) Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122:2049–2063
Szabo G, Bala S (2013) MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol 10:542–552
Kitano M, Bloomston PM (2016) Hepatic stellate cells and microRNAs in pathogenesis of liver fibrosis. J Clin Med 5. https://doi.org/10.3390/jcm5030038
MR MG, Staggs VS, Sharpe MR, Lee WM, Jaeschke H, Acute Liver Failure Study G (2014) Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 60:1336–1345
He Y, Feng D, Li M, Gao Y, Ramirez T, Cao H, Kim SJ, Yang Y, Cai Y, Ju C, Wang H, Li J, Gao B (2017) Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 66:220–234
Eguchi A, Kostallari E, Feldstein AE, Shah VH (2019) Extracellular vesicles, the liquid biopsy of the future. J Hepatol 70:1292–1294
Eguchi A, Feldstein AE (2018) Extracellular vesicles in non-alcoholic and alcoholic fatty liver diseases. Liver Res 2:30–34
Acknowledgments
We thank the UCSD IGM Genomics Center, especially Kristen Jepsen for siRNA sequencing advice and assistance and Rui Yan for mtDNA and TLR9-reporter assays. The authors would like to thank the UCSD/CMM electron microscopy facility, especially Timothy Meerloo, for TEM sample preparation and imaging. This EM facility is supported by NIH equipment grant 1S10OD023527. Proteomics work was performed in W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a Department of Energy (DOE) office of Biological and Environmental Research (BER) national user facility located at Pacific Northwest National Laboratory (PNNL).
Funding
The work was supported by NIH grants P50AA011999 (Pilot Project Program), R21 AA023574, and JSPS KAKENHI Grant Number JP16H06872 to AE; JSPS KAKENHI Grant Number JP17K09419 to YT; NIH grants U54HL108460 to JK and LOM; NIH grant P41 GM103493 to RDS; NIH grants R01 AA022489 and DK082451 to AEF; NIH grants P50AA011999 (Administrative and Animal Cores), R24AA012885 (Integrative Liver Cell Core), R01AA018663, U01AA027681 and VA grants I01BX001991 (VA Merit Review) and IK6BX004205 (Senior Research Career Scientist award) to HT.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The use and care of the animals was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Southern California.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 819 kb)
Rights and permissions
About this article
Cite this article
Eguchi, A., Yan, R., Pan, S.Q. et al. Comprehensive characterization of hepatocyte-derived extracellular vesicles identifies direct miRNA-based regulation of hepatic stellate cells and DAMP-based hepatic macrophage IL-1β and IL-17 upregulation in alcoholic hepatitis mice. J Mol Med 98, 1021–1034 (2020). https://doi.org/10.1007/s00109-020-01926-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01926-7