Abstract
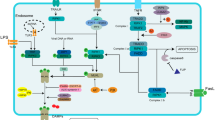
Necrosis with inflammation plays a crucial role in acute respiratory distress syndrome (ARDS). Receptor-interacting protein 3 (RIPK3) regulates a newly discovered programmed form of necrosis called necroptosis. However, the underlying mechanism of necroptosis in ARDS remains unknown. Thus, the purpose of this study was to examine the possible involvement of RIPK3 in ARDS-associated necroptosis. RIPK3 protein levels were found to be significantly elevated in the plasma and bronchoalveolar lavage fluid of ARDS patients. Next, we utilised a mouse model of severe ARDS induced with high-dose lipopolysaccharide and found that lung injury was mainly due to RIPK3-mixed lineage kinase domain-like pseudokinase (MLKL)-mediated necroptosis and endothelial dysfunction. The activation of RIPK3-MLKL by tumour necrosis factor receptor 1 (TNFR1) and TNFR1-associated death domain protein (TRADD) required catalytically active RIPK1 and the inhibition of Fas-associated protein with death domain (FADD)/caspase-8 catalytic activity. We further showed that the molecular chaperone heat shock protein 90 (Hsp90)/p23, as a novel RIPK3- and MLKL-interacting complex, played an important role in RIP-MLKL-mediated necroptosis, inflammation and endothelial dysfunction in the pulmonary vasculature, which resulted in ARDS. Collectively, the results of our study indicate that necroptosis is an important mechanism of cell death in ARDS and the inhibition of necroptosis may be a therapeutic intervention for ARDS.
Key messages
-
Lung injury in high-dose LPS-induced severe ARDS is mainly due to RIP3-MLKL-mediated necroptosis and endothelial dysfunction.
-
Chaperone HSP90/p23 is a novel RIP3- and MLKL-interacting complex in HPAECs.
-
HSP90/p23 is a novel RIP3- and MLKL-interacting complex in RIP-MLKL-mediated necroptosis, inflammation and endothelial dysfunction.








Similar content being viewed by others
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- cIAP1/2:
-
Cellular inhibitor of apoptosis 1/2
- ECs:
-
Endothelial cells
- FADD:
-
Fas-associated protein with death domain
- HPAECs:
-
Human pulmonary artery endothelial cells
- HSPs:
-
Heat shock proteins
- RIP3:
-
Receptor-interacting protein Kinase 3
- RIP1:
-
Receptor-interacting protein Kinase 1
- MLKL:
-
Mixed lineage kinase domain-like pseudokinase
- NSA:
-
Necrosulfonamide
- TNFR1:
-
Tumour necrosis factor receptor 1
- TRADD:
-
TNFR1-associated death domain protein
- TRAF2:
-
TNFR associated factor 2
- 17-AAG:
-
17-allylamino-17-demethoxygeldanamycin
References
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A, Slutsky AS, Vincent JL, Rubenfeld GD, Thompson BT, Ranieri VM (2012) The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 38:1573–1582
Tang PS, Mura M, Seth R, Liu M (2008) Acute lung injury and cell death: how many ways can cells die? Am J Phys Lung Cell Mol Phys 294:L632–L641
Wang L, Wang T, Li H, Liu Q, Zhang Z, Xie W, Feng Y, Socorburam T, Wu G, Xia Z et al (2016) Receptor interacting protein 3-mediated Necroptosis promotes lipopolysaccharide-induced inflammation and acute respiratory distress syndrome in mice. PLoS One 11:e0155723
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109:5322–5327
Al-Lamki RS, Lu W, Manalo P, Wang J, Warren AY, Tolkovsky AM, Pober JS, Bradley JR (2016) Tubular epithelial cells in renal clear cell carcinoma express high RIPK1/3 and show increased susceptibility to TNF receptor 1-induced necroptosis. Cell Death Dis 7:e2287
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459
Singla S, Sysol JR, Dille B, Jones N, Chen J, Machado RF (2017) Hemin causes lung microvascular endothelial barrier dysfunction by necroptotic cell death. Am J Respir Cell Mol Biol 57:307–314
Lv X, Wen T, Song J, Xie D, Wu L, Jiang X, Jiang P, Wen Z (2017) Extracellular histones are clinically relevant mediators in the pathogenesis of acute respiratory distress syndrome. Respir Res 18:165
Zhou H, Li D, Zhu P, Ma Q, Toan S, Wang J, Hu S, Chen Y, Zhang Y (2018) Inhibitory effect of melatonin on necroptosis via repressing the Ripk3-PGAM5-CypD-mPTP pathway attenuates cardiac microvascular ischemia-reperfusion injury. J Pineal Res 65:e12503
Carra S, Alberti S, Arrigo PA, Benesch JL, Benjamin IJ, Boelens W, Bartelt-Kirbach B, Brundel B, Buchner J, Bukau B, Carver JA, Ecroyd H, Emanuelsson C, Finet S, Golenhofen N, Goloubinoff P, Gusev N, Haslbeck M, Hightower LE, Kampinga HH, Klevit RE, Liberek K, Mchaourab HS, McMenimen K, Poletti A, Quinlan R, Strelkov SV, Toth ME, Vierling E, Tanguay RM (2017) The growing world of small heat shock proteins: from structure to functions. Cell Stress Chaperones 22:601–611
Urban MJ, Dobrowsky RT, Blagg BS (2012) Heat shock response and insulin-associated neurodegeneration. Trends Pharmacol Sci 33:129–137
Hoter A, El-Sabban ME, Naim HY (2018) The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci 19:e2560
Wang M, Shen A, Zhang C, Song Z, Ai J, Liu H, Sun L, Ding J, Geng M, Zhang A (2016) Development of heat shock protein (Hsp90) inhibitors to combat resistance to tyrosine kinase inhibitors through Hsp90-kinase interactions. J Med Chem 59:5563–5586
Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z (2015) A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci U S A 112:5017–5022
Li D, Li C, Li L, Chen S, Wang L, Li Q, Wang X, Lei X, Shen Z (2016) Natural product Kongensin a is a non-canonical HSP90 inhibitor that blocks RIP3-dependent Necroptosis. Cell Chem Biol 23:257–266
Park SY, Shim JH, Chae JI, Cho YS (2015) Heat shock protein 90 inhibitor regulates necroptotic cell death via down-regulation of receptor interacting proteins. Pharmazie 70:193–198
Jacobsen AV, Silke J (2016) The importance of being chaperoned: HSP90 and necroptosis. Cell Chem Biol 23:205–207
Pasparakis M, Vandenabeele P (2015) Necroptosis and its role in inflammation. Nature 517:311–320
Guo H, Kaiser WJ, Mocarski ES (2015) Manipulation of apoptosis and necroptosis signaling by herpesviruses. Med Microbiol Immunol 204:439–448
Bhargava M, Wendt CH (2012) Biomarkers in acute lung injury. Transl Res 159:205–217
Vasseur P, Devaure I, Sellier J, Delwail A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H, Lecron JC, Silvain C (2014) High plasma levels of the pro-inflammatory cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra in acute pancreatitis. Pancreatology 14:465–469
Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S (2013) Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A 110:E3109–E3118
Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL (2013) TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis 4:e465
Gonzales JN, Gorshkov B, Varn MN, Zemskova MA, Zemskov EA, Sridhar S, Lucas R, Verin AD (2014) Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am J Phys Lung Cell Mol Phys 306:L497–L507
Shen H, Liu C, Zhang D, Yao X, Zhang K, Li H, Chen G (2017) Role for RIP1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death Dis 8:e2641
Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F, Liu Y, Zheng W, Shang H, Zhang J, Zhang M, Wu H, Guo J, Zhang X, Hu X, Cao CM, Xiao RP (2016) CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med 22:175–182
Qing DY, Conegliano D, Shashaty MG, Seo J, Reilly JP, Worthen GS, Huh D, Meyer NJ, Mangalmurti NS (2014) Red blood cells induce necroptosis of lung endothelial cells and increase susceptibility to lung inflammation. Am J Respir Crit Care Med 190:1243–1254
Frantzeskaki F, Armaganidis A, Orfanos SE (2017) Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration 93:212–225
Gandhirajan RK, Meng S, Chandramoorthy HC, Mallilankaraman K, Mancarella S, Gao H, Razmpour R, Yang XF, Houser SR, Chen J, Koch WJ, Wang H, Soboloff J, Gill DL, Madesh M (2013) Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J Clin Invest 123:887–902
Siegmund D, Kums J, Ehrenschwender M, Wajant H (2016) Activation of TNFR2 sensitizes macrophages for TNFR1-mediated necroptosis. Cell Death Dis 7:e2375
Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M (2014) RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513:90–94
Saveljeva S, Mc Laughlin SL, Vandenabeele P, Samali A, Bertrand MJ (2015) Endoplasmic reticulum stress induces ligand-independent TNFR1-mediated necroptosis in L929 cells. Cell Death Dis 6:e1587
Newton K (2015) RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol 25:347–353
Dong W, Zhang M, Zhu Y, Chen Y, Zhao X, Li R, Zhang L, Ye Z, Liang X (2017) Protective effect of NSA on intestinal epithelial cells in a necroptosis model. Oncotarget 8:86726–86735
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111
Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M (2011) The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity 35:572–582
Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, Walsh CM (2011) Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A 108:15312–15317
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M (2011) FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477:330–334
Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J (2011) Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471:373–376
Lee EW, Kim JH, Ahn YH, Seo J, Ko A, Jeong M, Kim SJ, Ro JY, Park KM, Lee HW et al (2012) Ubiquitination and degradation of the FADD adaptor protein regulate death receptor-mediated apoptosis and necroptosis. Nat Commun 3:978
Taipale M, Jarosz DF, Lindquist S (2010) HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 11:515–528
Madon-Simon M, Grad I, Bayo P, Perez P, Picard D (2017) Defective glucocorticoid receptor signaling and keratinocyte-autonomous defects contribute to skin phenotype of mouse embryos lacking the Hsp90 co-chaperone p23. PLoS One 12:e0180035
Echtenkamp FJ, Gvozdenov Z, Adkins NL, Zhang Y, Lynch-Day M, Watanabe S, Peterson CL, Freeman BC (2016) Hsp90 and p23 molecular chaperones control chromatin architecture by maintaining the functional Pool of the RSC chromatin remodeler. Mol Cell 64:888–899
Funding
This work was supported by National Natural Science Foundation of China (contract grant numbers 81800047 to X.Y. 31820103007 and 31771276 to D.Z.),University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (contract grant numbers UNPYSCT-2018067 to X.Y.), Wu Liande Young Scientific Research Foundation of Harbin Medical University Daqing (contract grant number DQWLD20 to X.Y.) and Postdoctoral Research Funding (contract grant number LBH-Q19140 to X.Y.).
Author information
Authors and Affiliations
Contributions
Daling Zhu conceived the experiments. Xiufeng Yu, Mao Min, Xia Liu, Tingting Sheng, Tingting Li, Hao Yu and Xijuan Zhao conducted the experiments. Junting Zhang registered information on human samples and Xinxin Chen collected the plasma and bronchoalveolar lavage fluid (BALF) of human samples. Xiufeng Yu analysed and interpreted the data. Xiufeng Yu and Min Mao wrote the manuscript. Xiufeng Yu prepared the figures. All authors read and approved the final manuscript. Xiufeng Yu and Min Mao contribute equally for this study and were considered the co-first author. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The collection of human specimens was approved by the Biomedical Ethics Committee of the First Affiliated Hospital of Harbin Medical University, and the written informed consent were obtained from each patient.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, X., Mao, M., Liu, X. et al. A cytosolic heat shock protein 90 and co-chaperone p23 complex activates RIPK3/MLKL during necroptosis of endothelial cells in acute respiratory distress syndrome. J Mol Med 98, 569–583 (2020). https://doi.org/10.1007/s00109-020-01886-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01886-y