Abstract
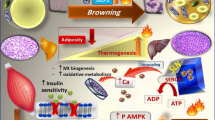
The postmenopausal state is associated with an increased risk of metabolic disorder including reduced energy expenditure and weight gain, leading to higher cardiovascular and cancer risks among other diseases. Mitochondrial-derived peptide (MOTS-c) is a 16–amino acid peptide encoded by mitochondrial DNA. Here, we showed that MOTS-c treatment in mice prevented ovariectomy-induced obesity and insulin resistance. After ovariectomy, low levels of estrogens increased fat mass overload and disturbed normal adipose function, forcing the development of insulin resistance. MOTS-c treatment increased brown fat activation and reduced OVX-induced fat accumulation and inflammatory invasion in white adipose tissue, which contributes to the lower level of fatty acid in serum and liver. Moreover, MOTS-c activated AMPK pathway to improve energy dissipation and insulin sensitivity. And a blocker of AMPK pathway was found to attenuate the role of MOTS-c in the regulation of adipocyte lipid metabolism. In conclusion, MOTS-c is a high potential candidate for chronic treatment of menopausal induced metabolic dysfunction.
Key messages
• MOTS-c prevents ovariectomy (OVX)-induced body weight gain and insulin resistance.
• MOTS-c reduces fat mass and suppresses inflammatory response under OVX condition.
• MOTS-c sustains the activity of the brown adipose under OVX condition.
• MOTS-c mediates AMPK pathway activation to control adipose metabolic homeostasis.






Similar content being viewed by others
References
Sharma K, Bansal M (2018) Association of age at menopause with post-menopausal symptoms, menarche age and other reproductive factors among rural females in Shimla, Himachal Pradesh. J Biosoc Sci 50:19–25
Pei J, Harakalova M, den Ruijter H, Pasterkamp G, Duncker DJ, Verhaar MC, Asselbergs FW, Cheng C (2017) Cardiorenal disease connection during post-menopause: the protective role of estrogen in uremic toxins induced microvascular dysfunction. Int J Cardiol 238:22–30
Zhu L, Brown WC, Cai Q, Krust A, Chambon P, McGuinness OP, Stafford JM (2013) Estrogen treatment after ovariectomy protects against fatty liver and may improve pathway-selective insulin resistance. Diabetes 62:424–434
Colvin GB, Whitmoyer DI, Sawyer CH (1969) Circadian sleep-wakefulness patterns in rats after ovariectomy and treatment with estrogen. Exp Neurol 25:616–625
Choi JS, Koh IU, Song J (2012) Genistein reduced insulin resistance index through modulating lipid metabolism in ovariectomized rats. Nutr Res 32:844–855
(2005) [Better metabolic control with patient-focused insulin administration]. Medizinische Monatsschrift fur Pharmazeuten 28: 366
Hoffmeister M, Raum E, Winter J, Chang-Claude J, Brenner H (2007) Hormone replacement therapy, body mass, and the risk of colorectal cancer among postmenopausal women from Germany. Br J Cancer 97:1486–1492
Vickers MR, MacLennan AH, Lawton B, Ford D, Martin J, Meredith SK, DeStavola BL, Rose S, Dowell A, Wilkes HC, Darbyshire JH, Meade TW, group W (2007) Main morbidities recorded in the women’s international study of long duration oestrogen after menopause (WISDOM): a randomised controlled trial of hormone replacement therapy in postmenopausal women. Bmj 335:239
Beranger GE, Pisani DF, Castel J, Djedaini M, Battaglia S, Amiaud J, Boukhechba F, Ailhaud G, Michiels JF, Heymann D, Luquet S, Amri EZ (2014) Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology 155:1340–1352
Kosikowska P, Lesner A (2016) Antimicrobial peptides (AMPs) as drug candidates: a patent review (2003-2015). Expert Opin Therapeut Patents 26:689–702
Malik V, Dhanjal JK, Kumari A, Radhakrishnan N, Singh K, Sundar D (2017) Function and structure-based screening of compounds, peptides and proteins to identify drug candidates. Methods 131:10–21
Lee C, Kim KH, Cohen P (2016) MOTS-c: a novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic Biol Med 100:182–187
Qin Q, Delrio S, Wan J, Jay Widmer R, Cohen P, Lerman LO, Lerman A (2018) Downregulation of circulating MOTS-c levels in patients with coronary endothelial dysfunction. Int J Cardiol 254:23–27
Cataldo LR, Fernandez-Verdejo R, Santos JL, Galgani JE (2018) Plasma MOTS-c levels are associated with insulin sensitivity in lean but not in obese individuals. J Investig Med: Off Publ Am Fed Clin Res 66:1019–1022
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, Kim SJ, Mehta H, Hevener AL, de Cabo R, Cohen P (2015) The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab 21:443–454
Ming W, Lu G, Xin S, Huanyu L, Yinghao J, Xiaoying L, Chengming X, Banjun R, Li W, Zifan L (2016) Mitochondria related peptide MOTS-c suppresses ovariectomy-induced bone loss via AMPK activation. Biochem Biophys Res Commun 476:412–419
El Maghraoui A, Rezqi A, El Mrahi S, Sadni S, Ghozlani I, Mounach A (2014) Osteoporosis, vertebral fractures and metabolic syndrome in postmenopausal women. BMC Endocr Disord 14:93
Ambrosi TH, Scialdone A, Graja A, Gohlke S, Jank AM, Bocian C, Woelk L, Fan H, Logan DW, Schurmann A, Saraiva LR, Schulz TJ (2017) Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell 20: 771–784 e776. DOI https://doi.org/10.1016/j.stem.2017.02.009
Choudhury AB, Sarkar PD, Sakalley DK, Petkar SB (2014) Role of adiponectin in mediating the association of osteocalcin with insulin resistance and type 2 diabetes: a cross sectional study in pre- and post-menopausal women. Arch Physiol Biochem 120:73–79
Tanna N, Patel K, Moore AE, Dulnoan D, Edwards S, Hampson G (2017) The relationship between circulating adiponectin, leptin and vaspin with bone mineral density (BMD), arterial calcification and stiffness: a cross-sectional study in post-menopausal women. J Endocrinol Investig 40:1345–1353
Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP, Liao EY, Luo XH (2010) Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Investig 33:707–711
Wang X, Wei W, Krzeszinski JY, Wang Y, Wan Y (2015) A liver-bone endocrine relay by IGFBP1 promotes osteoclastogenesis and mediates FGF21-induced bone resorption. Cell Metab 22:811–824
Andersen B, Straarup EM, Heppner KM, Takahashi DL, Raffaele V, Dissen GA, Lewandowski K, Bodvarsdottir TB, Raun K, Grove KL, Kievit P (2018) FGF21 decreases body weight without reducing food intake or bone mineral density in high-fat fed obese rhesus macaque monkeys. Int J Obes 42:1151–1160
Yen K, Lee C, Mehta H, Cohen P (2013) The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol 50:R11–R19
Qin Q, Jin J, He F, Zheng Y, Li T, Zhang Y, He J (2018) Humanin promotes mitochondrial biogenesis in pancreatic MIN6 beta-cells. Biochem Biophys Res Commun 497:292–297
Han K, Jia N, Zhong Y, Shang X (2018) S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem 119:3111–3117
Zhai D, Ye Z, Jiang Y, Xu C, Ruan B, Yang Y, Lei X, Xiang A, Lu H, Zhu Z, Yan Z, Wei D, Li Q, Wang L, Lu Z (2017) MOTS-c peptide increases survival and decreases bacterial load in mice infected with MRSA. Mol Immunol 92:151–160
Funding
This research was supported by the National Natural Science Foundation of China (NSF: 31571215; NSF: 31270843, NSF: 81330045; NSF: 81730053) and the Military Logistics Research Project (AWS14L008; AWS16J022).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 228 kb)
Rights and permissions
About this article
Cite this article
Lu, H., Wei, M., Zhai, Y. et al. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J Mol Med 97, 473–485 (2019). https://doi.org/10.1007/s00109-018-01738-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-018-01738-w