Abstract
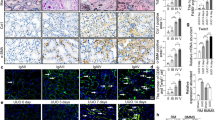
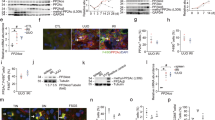
TIMAP (TGFβ-inhibited membrane-associated protein) is an endothelium-enriched TGFβ downstream protein and structurally belongs to the targeting subunit of myosin phosphatase; however, the mechanism of TGFβ repressing TIMAP and its functional relevance to TGFβ bioactivity remain largely unknown. Here, we report that TIMAP is reduced in TGFβ-elevated mouse fibrotic kidney and highly expressed in macrophages. TGFβ repression of TIMAP is associated with HDAC3 upregulation and its recruitment by Smad2/3 at the Smad binding element on TIMAP promoter, whereas specific HDAC3 inhibition reversed the TIMAP repression, suggesting that TGFβ transcriptionally downregulates TIMAP through HDAC3-associated Smad signaling. Further investigation showed that TIMAP over-expression interrupted TGFβ-associated Smad signaling and TIMAP repression by TGFβ correlated with TGFβ-induced macrophage M2 polarization markers, migration, and phagocytosis—the processes promoted by phosphorylation of the putative TIMAP substrate myosin light chain (MLC). Consistently, TIMAP dephosphorylated MLC in macrophages and TGFβ induced macrophage migration and phagocytosis in TIMAP- and MLC phosphorylation-dependent manners, suggesting that TIMAP dephosphorylation of MLC constitutes an essential regulatory loop mitigating TGFβ-associated macrophage M2 phenotypic activities. Given that hyperactive TGFβ often causes excessive macrophage phagocytic activities potentially leading to various chronic disorders, the strategies targeting HDAC3/TIMAP axis might improve TGFβ-associated pathological processes.
Key message
-
TIMAP is enriched in the endothelium and highly expressed in macrophages.
-
TIMAP is suppressed by TGFβ via HDAC3-associated Smad signaling.
-
TIMAP inhibits TGFβ signaling and TGFβ-associated macrophage M2 polarization.
-
TIMAP dephosphorylation of MLC counteracts TGFβ-induced macrophage phagocytosis.








Similar content being viewed by others
References
Cao W, Mattagajasingh SN, Xu H, Kim K, Fierlbeck W, Deng J, Lowenstein CJ, Ballermann BJ (2002) TIMAP, a novel CAAX box protein regulated by TGF-beta1 and expressed in endothelial cells. Am J Physiol Cell Physiol 283: C327–C337
Hawinkels LJ, Ten Dijke P (2011) Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 29:140–152
Poirier C, Gorshkov BA, Zemskova MA, Bogatcheva NV, Verin AD (2011) TIMAP protects endothelial barrier from LPS-induced vascular leakage and is down-regulated by LPS. Respir Physiol Neurobiol 179:334–337
Kim K, Li L, Kozlowski K, Suh HS, Cao W, Ballermann BJ (2005) The protein phosphatase-1 targeting subunit TIMAP regulates LAMR1 phosphorylation. Biochem Biophys Res Commun 338: 1327–1334
Li L, Kozlowski K, Wegner B, Rashid T, Yeung T, Holmes C, Ballermann BJ (2007) Phosphorylation of TIMAP by glycogen synthase kinase-3beta activates its associated protein phosphatase 1. J Biol Chem 282: 25960–25969
Csortos C, Czikora I, Bogatcheva NV, Adyshev DM, Poirier C, Olah G, Verin AD (2008) TIMAP is a positive regulator of pulmonary endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 295:L440–L450
Boratko A, Gergely P, Csortos C (2013) RACK1 is involved in endothelial barrier regulation via its two novel interacting partners. Cell Commun Signal 11:2
Obeidat M, Li L, Ballermann BJ (2014) TIMAP promotes angiogenesis by suppressing PTEN-mediated Akt inhibition in human glomerular endothelial cells. Am J Physiol Renal Physiol 307:F623–F633
Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A (2010) Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell 39:373–384
Simonsson M, Kanduri M, Gronroos E, Heldin CH, Ericsson J (2006) The DNA binding activities of Smad2 and Smad3 are regulated by coactivator-mediated acetylation. J Biol Chem 281:39870–39880
Nomura T, Khan MM, Kaul SC, Dong HD, Wadhwa R, Colmenares C, Kohno I, Ishii S (1999) Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev 13:412–423
Tabata T, Kokura K, Ten Dijke P, Ishii S (2009) Ski co-repressor complexes maintain the basal repressed state of the TGF-beta target gene, SMAD7, via HDAC3 and PRMT5. Genes Cells 14:17–28
Tavian M, Cortes F, Robin C, Schiavon V, Hallais MF, Coulombel L, Charbord P, Labastie MC, Peault B (2000) The hemangioblast, common precursor of endothelial and hematopoietic cells. Transfus Clin Biol 7:238–241
Bohdanowicz M, Grinstein S (2013) Role of phospholipids in endocytosis, phagocytosis, and macropinocytosis. Physiol Rev 93:69–106
Niedergang F, Chavrier P (2004) Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol 16:422–428
Stuart LM, Ezekowitz RA (2005) Phagocytosis: elegant complexity. Immunity 22:539–550
Sun C, MH W, Yuan SY (2011) Nonmuscle myosin light-chain kinase deficiency attenuates atherosclerosis in apolipoprotein E-deficient mice via reduced endothelial barrier dysfunction and monocyte migration. Circulation 124:48–57
Sharma L, Wu W, Dholakiya SL, Gorasiya S, Wu J, Sitapara R, Patel V, Wang M, Zur M, Reddy S et al (2014) Assessment of phagocytic activity of cultured macrophages using fluorescence microscopy and flow cytometry. Methods Mol Biol 1172:137–145
Shopik MJ, Li L, Luu HA, Obeidat M, Holmes CF, Ballermann BJ (2013) Multi-directional function of the protein phosphatase 1 regulatory subunit TIMAP. Biochem Biophys Res Commun 435: 567–573
Qin T, Du R, Huang F, Yin S, Yang J, Qin S, Cao W (2016) Sinomenine activation of Nrf2 signaling prevents hyperactive inflammation and kidney injury in a mouse model of obstructive nephropathy. Free Radic Biol Med 92:90–99
Qin S, Du R, Yin S, Liu X, Xu G, Cao W (2015) Nrf2 is essential for the anti-inflammatory effect of carbon monoxide in LPS-induced inflammation. Inflamm Res 64:537–548
Cao W, Bao C, Padalko E, Lowenstein CJ (2008) Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. J Exp Med 205:1491–1503
Yin S, Cao W (2015) TLR signaling induces Nrf2 pathway activation through p62-triggered Keap1 degradation. Mol Cell Biol. doi:10.1128/MCB.00105-15
Konopski Z, Fandrem J, Seljelid R, Eskeland T (1995) Interferon-gamma inhibits endocytosis of soluble animated beta-1,3-D-glucan and neutral red in mouse peritoneal macrophages. J Interf Cytokine Res 15:597–603
Fleetwood AJ, Lawrence T, Hamilton JA, Cook AD (2007) Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J Immunol 178:5245–5252
Gitik M, Reichert F, Rotshenker S (2010) Cytoskeleton plays a dual role of activation and inhibition in myelin and zymosan phagocytosis by microglia. FASEB J 24:2211–2221
Fuentes AL, Millis L, Vapenik J, Sigola L (2014) Lipopolysaccharide-mediated enhancement of zymosan phagocytosis by RAW 264.7 macrophages is independent of opsonins, laminarin, mannan, and complement receptor 3. J Surg Res 189:304–312
Klahr S, Morrissey J (2003) Obstructive nephropathy and renal fibrosis: the role of bone morphogenic protein-7 and hepatocyte growth factor. Kidney Int Suppl 87:S105–S112
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Li C, Yang G, Ruan J (2012) Sphingosine kinase-1/sphingosine-1-phosphate receptor type 1 signalling axis is induced by transforming growth factor-beta1 and stimulates cell migration in RAW264.7 macrophages. Biochem Biophys Res Commun 426:415–420
Levin R, Grinstein S, Schlam D (2015) Phosphoinositides in phagocytosis and macropinocytosis. Biochim Biophys Acta 1851:805–823
Ashcroft GS (1999) Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect 1:1275–1282
Derynck R, Zhang YE (2003) Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 425:577–584
Braga TT, Agudelo JS, Camara NO (2015) Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol 6:602
Liu N, Zhuang S (2015) Treatment of chronic kidney diseases with histone deacetylase inhibitors. Front Physiol 6:121
Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S (2009) Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297:F996–F1005
Massague J, Wotton D (2000) Transcriptional control by the TGF-beta/Smad signaling system. EMBO J 19:1745–1754
Ryu JK, Kim WJ, Choi MJ, Park JM, Song KM, Kwon MH, Das ND, Kwon KD, Batbold D, Yin GN et al (2013) Inhibition of histone deacetylase 2 mitigates profibrotic TGF-beta1 responses in fibroblasts derived from Peyronie’s plaque. Asian J Androl 15:640–645
Glenisson W, Castronovo V, Waltregny D (2007) Histone deacetylase 4 is required for TGFbeta1-induced myofibroblastic differentiation. Biochim Biophys Acta 1773:1572–1582
Shan B, Yao TP, Nguyen HT, Zhuo Y, Levy DR, Klingsberg RC, Tao H, Palmer ML, Holder KN, Lasky JA (2008) Requirement of HDAC6 for transforming growth factor-beta1-induced epithelial-mesenchymal transition. J Biol Chem 283:21065–21073
Liu L, Lin W, Zhang Q, Cao W, Liu Z (2015) TGF-beta induces miR-30d down-regulation and podocyte injury through Smad2/3 and HDAC3-associated transcriptional repression. J Mol Med (Berl). doi:10.1007/s00109-015-1340-9
Jiang X, Ye X, Guo W, Lu H, Gao Z (2014) Inhibition of HDAC3 promotes ligand-independent PPARgamma activation by protein acetylation. J Mol Endocrinol 53:191–200
Bright MD, Frankel G (2011) PAK4 phosphorylates myosin regulatory light chain and contributes to Fcgamma receptor-mediated phagocytosis. Int J Biochem Cell Biol 43:1776–1781
Deery WJ, Heath JP (1993) Phagocytosis induced by thyrotropin in cultured thyroid cells is associated with myosin light chain dephosphorylation and stress fiber disruption. J Cell Biol 122:21–37
Mansfield PJ, Shayman JA, Boxer LA (2000) Regulation of polymorphonuclear leukocyte phagocytosis by myosin light chain kinase after activation of mitogen-activated protein kinase. Blood 95:2407–2412
Ito M, Nakano T, Erdodi F, Hartshorne DJ (2004) Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259:197–209
Ricevuti G (1997) Host tissue damage by phagocytes. Ann N Y Acad Sci 832:426–448
Laskin DL, Sunil VR, Gardner CR, Laskin JD (2011) Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 51:267–288
Meng XM, Nikolic-Paterson DJ, Lan HY (2016) TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. doi:10.1038/nrneph.2016.48
Acknowledgments
This work was supported by research grants from the National Nature Science Foundation of China (81271301, 81470940, and 81670672) to W. Cao.
Authors’ contributions
JY performed most experiments and participated in manuscript writing; SY, FB, LL, and QT assisted in animal work, data collection, and some experiments. HW provided critical advice; WC designed and conceived the project, analyzed and arranged results, and wrote the manuscript. All the authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Yang, J., Yin, S., Bi, F. et al. TIMAP repression by TGFβ and HDAC3-associated Smad signaling regulates macrophage M2 phenotypic phagocytosis. J Mol Med 95, 273–285 (2017). https://doi.org/10.1007/s00109-016-1479-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1479-z