Abstract
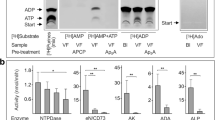
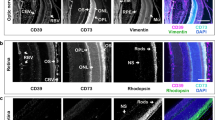
Clear signaling roles for ATP and adenosine have been established in all tissues, including the eye. The magnitude of signaling responses is governed by networks of enzymes; however, little is known about the regulatory mechanisms of purinergic signaling in the eye. By employing thin-layer chromatographic assays with 3H-labeled substrates, this study aimed to evaluate the role of nucleotide homeostasis in the pathogenesis of vitreoretinal diseases in humans. We have identified soluble enzymes ecto-5′-nucleotidase/CD73, adenylate kinase-1, and nucleoside diphosphate kinase in the vitreous fluid that control active cycling between pro-inflammatory ATP and anti-inflammatory adenosine. Strikingly, patients with proliferative form of diabetic retinopathy (DR) had higher adenylate kinase activity and ATP concentration, when compared to non-proliferative DR eyes and non-diabetic controls operated for rhegmatogenous retinal detachment, macular hole, and pucker. The non-parametric correlation analysis revealed positive correlations between intravitreal adenylate kinase and concentrations of ATP, ADP, and other angiogenic (angiopoietins-1 and -2), profibrotic (transforming growth factor-β1), and proteolytic (matrix metalloproteinase-9) factors but not erythropoietin and VEGF. Immunohistochemical staining of postmortem human retina additionally revealed selective expression of ecto-5′-nucleotidase/CD73 on the rod-and-cone-containing photoreceptor cells. Collectively, these findings provide novel insights into the regulatory mechanisms that influence purinergic signaling in diseased eye and open up new possibilities in the development of enzyme-targeted therapeutic approaches for prevention and treatment of DR.
Key message
-
Ecto-5′-nucleotidase/CD73 and adenylate kinase-1 circulate in human vitreous fluid.
-
Adenylate kinase activity is high in diabetic eyes with proliferative retinopathy.
-
Diabetic eyes display higher intravitreal ATP/ADP ratio than non-diabetic controls.
-
Soluble adenylate kinase maintains resynthesis of inflammatory ATP in diabetic eyes






Similar content being viewed by others
References
Caprara C, Grimm C (2012) From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res 31:89–119
Loukovaara S, Gucciardo E, Repo P, Vihinen H, Lohi J, Jokitalo E, Salven P, Lehti K (2015) Indications of lymphatic endothelial differentiation and endothelial progenitor cell activation in the pathology of proliferative diabetic retinopathy. Acta Ophthalmol 93:512–523
Simo R, Hernandez C (2015) Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 48:160–180
Stitt AW, Curtis TM, Chen M, Medina RJ, McKay GJ, Jenkins A, Gardiner TA, Lyons TJ, Hammes HP, Simo R, et al. (2016) The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res 51:156–186
Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet 376:124–136
Loukovaara S, Robciuc A, Holopainen JM, Lehti K, Pessi T, Liinamaa J, Kukkonen KT, Jauhiainen M, Koli K, Keski-Oja J, et al. (2013) Ang-2 upregulation correlates with increased levels of MMP-9, VEGF, EPO and TGFbeta1 in diabetic eyes undergoing vitrectomy. Acta Ophthalmol 91:531–539
Loukovaara S, Nurkkala H, Tamene F, Gucciardo E, Liu X, Repo P, Lehti K, Varjosalo M (2015) Quantitative proteomics analysis of vitreous humor from diabetic retinopathy patients. J Proteome Res 14:5131–5143
Ghiardi GJ, Gidday JM, Roth S (1999) The purine nucleoside adenosine in retinal ischemia-reperfusion injury. Vis Res 39:2519–2535
Crooke A, Guzman-Aranguez A, Peral A, Abdurrahman MK, Pintor J (2008) Nucleotides in ocular secretions: their role in ocular physiology. Pharmacol Ther 119:55–73
Guzman-Aranguez A, Santano C, Martin-Gil A, Fonseca B, Pintor J (2013) Nucleotides in the eye: focus on functional aspects and therapeutic perspectives. J Pharmacol Exp Ther 345:331–341
Sanderson J, Dartt DA, Trinkaus-Randall V, Pintor J, Civan MM, Delamere NA, Fletcher EL, Salt TE, Grosche A, Mitchell CH (2014) Purines in the eye: recent evidence for the physiological and pathological role of purines in the RPE, retinal neurons, astrocytes, Muller cells, lens, trabecular meshwork, cornea and lacrimal gland. Exp Eye Res 127:270–279
Reichenbach A, Bringmann A (2016) Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology 104:194–211
Guzman-Aranguez A, Crooke A, Peral A, Hoyle CH, Pintor J (2007) Dinucleoside polyphosphates in the eye: from physiology to therapeutics. Prog Retin Eye Res 26:674–687
Zhong Y, Yang Z, Huang WC, Luo X (2013) Adenosine, adenosine receptors and glaucoma: an updated overview. Biochim Biophys Acta 1830:2882–2890
Lu W, Hu H, Sevigny J, Gabelt BT, Kaufman PL, Johnson EC, Morrison JC, Zode GS, Sheffield VC, Zhang X, et al. (2015) Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye. Invest Ophthalmol Vis Sci 56:3075–3083
Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, Takeda A, Ikeda Y, Enaida H, Sakamoto T, et al. (2013) Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One 8:e53338
Loukovaara S, Sahanne S, Jalkanen S, Yegutkin GG (2015) Increased intravitreal adenosine 5′-triphosphate, adenosine 5′-diphosphate and adenosine 5′-monophosphate levels in patients with proliferative diabetic retinopathy. Acta Ophthalmol 93:67–73
Burnstock G, Novak I (2013) Purinergic signalling and diabetes. Purinergic Signal 9:307–324
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367:2322–2333
Zimmermann H, Zebisch M, Strater N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502
Yegutkin GG (2014) Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49:473–497
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13:852–869
Idzko M, Ferrari D, Riegel AK, Eltzschig HK (2014) Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 124:1029–1037
Newman EA (2015) Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos Trans R Soc Lond Ser B Biol Sci 370
Iandiev I, Wurm A, Pannicke T, Wiedemann P, Reichenbach A, Robson SC, Zimmermann H, Bringmann A (2007) Ectonucleotidases in Muller glial cells of the rodent retina: involvement in inhibition of osmotic cell swelling. Purinergic Signal 3:423–433
Lu W, Reigada D, Sevigny J, Mitchell CH (2007) Stimulation of the P2Y1 receptor up-regulates nucleoside-triphosphate diphosphohydrolase-1 in human retinal pigment epithelial cells. J Pharmacol Exp Ther 323:157–164
Li A, Leung CT, Peterson-Yantorno K, Stamer WD, Civan MM (2011) Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci 52:7996–8005
Yegutkin GG, Samburski SS, Jalkanen S (2003) Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J 17:1328–1330
Helenius M, Jalkanen S, Yegutkin GG (2012) Enzyme-coupled assays for simultaneous detection of nanomolar ATP, ADP, AMP, adenosine, inosine and pyrophosphate concentrations in extracellular fluids. Biochim Biophys Acta 1823:1967–1975
Yegutkin GG, Henttinen T, Jalkanen S (2001) Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J 15:251–260
Yegutkin GG, Wieringa B, Robson SC, Jalkanen S (2012) Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. FASEB J 26:3875–3883
Donaldson SH, Picher M, Boucher RC (2002) Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am J Respir Cell Mol Biol 26:209–215
Westfall DP, Todorov LD, Mihaylova-Todorova ST (2002) ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther 303:439–444
Eberle D, Schubert S, Postel K, Corbeil D, Ader M (2011) Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest Ophthalmol Vis Sci 52:6462–6471
Janssen E, Kuiper J, Hodgson D, Zingman LV, Alekseev AE, Terzic A, Wieringa B (2004) Two structurally distinct and spatially compartmentalized adenylate kinases are expressed from the AK1 gene in mouse brain. Mol Cell Biochem:256–257
Park H, Kam TI, Kim Y, Choi H, Gwon Y, Kim C, Koh JY, Jung YK (2012) Neuropathogenic role of adenylate kinase-1 in Abeta-mediated tau phosphorylation via AMPK and GSK3beta. Hum Mol Genet 21:2725–2737
Notari L, Morelli A, Pepe IM (2003) Studies on adenylate kinase isoform bound to disk membranes of the rod outer segment of bovine retina. Photochem Photobiol Sci 2:1299–1302
Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T (2011) Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol 179:2798–2809
Aplin FP, Vessey KA, Luu CD, Guymer RH, Shepherd RK, Fletcher EL (2016) Retinal changes in an ATP-induced model of retinal degeneration. Front Neuroanat 10:46
Flores NA, Stavrou BM, Sheridan DJ (1999) The effects of diadenosine polyphosphates on the cardiovascular system. Cardiovasc Res 42:15–26
Koso H, Minami C, Tabata Y, Inoue M, Sasaki E, Satoh S, Watanabe S (2009) CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest Ophthalmol Vis Sci 50:5411–5418
Lakowski J, Gonzalez-Cordero A, West EL, Han YT, Welby E, Naeem A, Blackford SJ, Bainbridge JW, Pearson RA, Ali RR, et al. (2015) Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells 33:2469–2482
de Smet MD, Gad Elkareem AM, Zwinderman AH (2013) The vitreous, the retinal interface in ocular health and disease. Ophthalmologica 230:165–178
Moeckel D, Jeong SS, Sun X, Broekman MJ, Nguyen A, Drosopoulos JH, Marcus AJ, Robson SC, Chen R, Abendschein D (2014) Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med 6:248ra105
Mercier N, Kiviniemi TO, Saraste A, Miiluniemi M, Silvola J, Jalkanen S, Yegutkin GG (2012) Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. Am J Pathol 180:419–428
Yegutkin GG, Helenius M, Kaczmarek E, Burns N, Jalkanen S, Stenmark K, Gerasimovskaya EV (2011) Chronic hypoxia impairs extracellular nucleotide metabolism and barrier function in pulmonary artery vasa vasorum endothelial cells. Angiogenesis 14:503–513
Straub A, Krajewski S, Hohmann JD, Westein E, Jia F, Bassler N, Selan C, Kurz J, Wendel HP, Dezfouli S, et al. (2011) Evidence of platelet activation at medically used hypothermia and mechanistic data indicating ADP as a key mediator and therapeutic target. Arterioscler Thromb Vasc Biol 31:1607–1616
Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31:217–233
Acknowledgments
This work was supported by grants from the Academy of Finland, the Sigrid Juselius Foundation, the European Community’s Seventh Framework Program (FP7/2007–2013; grant agreement no. 602200) (SJ and GGY), the Finnish Eye Foundation, the Eye and Tissue Bank Foundation, the Mary and Georg C. Ehrnrooth Foundation, the Nissi Foundation, the Friends of the Blind, and HUCH Clinical Research Grants (TYH2016230 after TYH1325) (SL). We are grateful to Professors Be Wieringa and Jean Sevigny for providing the antibodies. We thank Professor Pier Enrico Gallenga and Drs. Giuseppe Lattanzio, Markku Kallajoki, and Maria Gardber for their expert help in examining the histological samples. We also thank Sari Mäki and Teija Kanasuo for their technical assistance, Seija Rusanen for drawing the schematic eye images, and Ruth Fair-Mäkelä for the revision of the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 859 kb)
Rights and permissions
About this article
Cite this article
Loukovaara, S., Sandholm, J., Aalto, K. et al. Deregulation of ocular nucleotide homeostasis in patients with diabetic retinopathy. J Mol Med 95, 193–204 (2017). https://doi.org/10.1007/s00109-016-1472-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1472-6