Abstract
Cumulative evidence suggests the importance of organelle homeostasis in regulating metabolic functions in response to various cellular stresses. Particularly, the dynamism and health of the mitochondria-peroxisome network through fission and fusion are essential for cellular function; dysfunctional dynamism underlies the pathogenesis of several degenerative diseases including Parkinson’s disease. Here, we investigated the role of Fis1 in cartilage homeostasis and its relevance to osteoarthritis (OA). We found that Fis1 is significantly suppressed in human OA chondrocytes compared to that in normal chondrocytes. Fis1 depletion through siRNA induced peroxisomal dysfunction. Moreover, Fis1 suppression altered miRNA profiles, especially those implicated in lysosomal regulation. Lysosomal destruction using LAMP-1-specific targeted nanorods or lysosomal dysfunction through chloroquine treatment resulted in enhanced chondrocyte apoptosis and/or suppression of autophagy. Accordingly, lysosomal activity and autophagy were severely decreased in OA chondrocytes despite abundant LAMP-1-positive organelles. Moreover, Fis1 morpholino-injected zebrafish embryos displayed lysosome accumulation, mitochondrial dysfunction, and peroxisome reduction. Collectively, these data suggest interconnected links among Fis1-modulated miRNA, lysosomes, and autophagy, which contributes to chondrocyte survival/apoptosis. This study represents the first functional study of Fis1 with its pathological relevance to OA. Our data suggest a new target for controlling cartilage-degenerative diseases, such as OA.
Key message
-
Fis1 suppression in OA chondrocytes induces accumulation and inhibition of lysosomes.
-
Fis1 suppression alters miRNAs, especially those implicated in lysosomal regulation.
-
Lysosomal destruction results in chondrocyte apoptosis and suppression of autophagy.
-
Fis1 depletion in zebrafish causes lysosome accumulation, mitochondrial dysfunction, and peroxisome reduction.
-
This is the first functional study of Fis1 and its pathological relevance to OA.






Similar content being viewed by others
Change history
19 November 2019
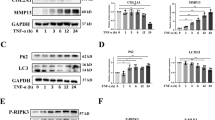
In Fig. 1C, the wrong si-control image was used by mistake.
19 November 2019
In Fig. 1C, the wrong si-control image was used by mistake.
References
Nunnari J, Walter P (1996) Regulation of organelle biogenesis. Cell 84:389–394
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516
Mishra P, Chan DC (2014) Mitochondrial dynamics and inheritance during cell division, development and disease. Nat Rev Mol Cell Biol 15:634–646
Parcellier A, Tintignac LA, Zhuravleva E, Hemmings BA (2008) PKB and the mitochondria: AKTing on apoptosis. Cell Signal 20:21–30
Sukhorukov VM, Dikov D, Reichert AS, Meyer-Hermann M (2012) Emergence of the mitochondrial reticulum from fission and fusion dynamics. PLoS Comput Biol 8:e1002745
Koike M, Nojiri H, Ozawa Y, Watanabe K, Muramatsu Y, Kaneko H, Morikawa D, Kobayashi K, Saita Y, Sasho T et al (2015) Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci Rep 5:11722
Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R (2015) Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheumatol 67:2141–2153
López de Figueroa P, Lotz MK, Blanco FJ, Caramés B (2015) Autophagy activation and protection from mitochondrial dysfunction in human chondrocytes. Arthritis Rheumatol 201567:966–976
Dave M, Attur M, Palmer G, Al-Mussawir HE, Kennish L, Patel J, Abramson SB (2014) The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum 58:2786–2797
Wu L, Liu H, Li L, Liu H, Cheng Q, Li H, Huang H (2014) Mitochondrial pathology in osteoarthritic chondrocytes. Curr Drug Targets 15:710–719
Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160:189–200
Loson OC, Song Z, Chen H, Chan DC (2013) Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell 24:659–667
Qian W, Choi S, Gibson GA, Watkins SC, Bakkenist CJ, Van Houten B (2012) Mitochondrial hyperfusion induced by loss of the fission protein Drp1 causes ATM-dependent G2/M arrest and aneuploidy through DNA replication stress. J Cell Sci 125:5745–5757
Waterham HR, Ebberink MS (2012) Genetics and molecular basis of human peroxisome biogenesis disorders. Biochim Biophys Acta 1822:1430–1441
Wanders RJ (2004) Peroxisomes, lipid metabolism, and peroxisomal disorders. Mol Genet Metab 83:16–27
Koepke JI, Nakrieko KA, Wood CS, Boucher KK, Terlecky LJ, Walton PA, Terlecky SR (2007) Restoration of peroxisomal catalase import in a model of human cellular aging. Traffic 8:1590–1600
Fransen M, Nordgren M, Wang B, Apanasets O (2012) Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochim Biophys Acta 1822:1363–1373
Delille HK, Agricola B, Guimaraes SC, Borta H, Luers GH, Fransen M, Schrader M (2010) Pex11pbeta-mediated growth and division of mammalian peroxisomes follows a maturation pathway. J Cell Sci 123:2750–2762
Li X, Gould SJ (2002) PEX11 promotes peroxisome division independently of peroxisome metabolism. J Cell Biol 156:643–651
Li X, Gould SJ (2003) The dynamin-like GTPase DLP1 is essential for peroxisome division and is recruited to peroxisomes in part by PEX11. J Biol Chem 278:17012–17020
Li X, Baumgart E, Morrell JC, Jimenez-Sanchez G, Valle D, Gould SJ (2002) PEX11 beta deficiency is lethal and impairs neuronal migration but does not abrogate peroxisome function. Mol Cell Biol 22:4358–4365
Baumgart E, Vanhorebeek I, Grabenbauer M, Borgers M, Declercq PE, Fahimi HD, Baes M (2001) Mitochondrial alterations caused by defective peroxisomal biogenesis in a mouse model for Zellweger syndrome (PEX5 knockout mouse). Am J Pathol 159:1477–1494
Kim D, Song J, Ahn C, Kang Y, Chun CH, Jin EJ (2014) Peroxisomal dysfunction is associated with up-regulation of apoptotic cell death via miR-223 induction in knee osteoarthritis patients with type 2 diabetes mellitus. Bone 64:124–131
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Reddy JK, Mannaerts GP (1994) Peroxisomal lipid metabolism. Annu Rev Nutr 14:343–370
Dirkx R, Vanhorebeek I, Martens K, Schad A, Grabenbauer M, Fahimi D, Declercq P, Van Veldhoven PP, Baes M (2005) Absence of peroxisomes in mouse hepatocytes causes mitochondrial and ER abnormalities. Hepatology 41:868–878
Yekta S, Shih IH, Bartel DP (2004) MicroRNA-directed cleavage of HOXB8 mRNA. Science 304:594–596
Djuranovic S, Nahvi A, Green R (2012) miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336:237–240
Okamoto K (2014) Organellophagy: eliminating cellular building blocks via selective autophagy. J Cell Biol 205:435–445
Eskelinen EL, Illert AL, Tanaka Y, Schwarzmann G, Blanz J, von Figura K, Saftig P (2002) Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell 13:3355–3368
Dominguez-Bautista JA, Klinkenberg M, Brehm N, Subramaniam M, Kern B, Roeper J, Auburger G, Jendrach M (2015) Loss of lysosome-associated membrane protein 3 (LAMP3) enhances cellular vulnerability against proteasomal inhibition. Eur J Cell Biol 94:148–161
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) [2013R1A2A2A01067194, 2016R1A2B4010577, 2011-0030130] to E-J Jin and NRF-2014M3A9D8034463 to Choe S-K and Park RK.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study was approved by the institutional review boards (Wonkwang University Ethics Committees, no. WKUH 1519) and performed in compliance with the Helsinki Declaration.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Dongkyun Kim and Jinsoo Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, D., Song, J., Kang, Y. et al. Fis1 depletion in osteoarthritis impairs chondrocyte survival and peroxisomal and lysosomal function. J Mol Med 94, 1373–1384 (2016). https://doi.org/10.1007/s00109-016-1445-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1445-9