Abstract
Experimental autoimmune myocarditis (EAM) is an inflammatory cardiac disease driven by autoantigen-specific CD4+ T cells. Th17 and Treg cells are crucial participants in immune response. A wide variety of immune disorders are associated with Th17/Treg imbalance. MicroRNA-155 (miR-155) is a pivotal regulator of the immune system. However, the modulatory effect of miR-155 on Th17/Treg immune response during EAM is unknown. Our study aims to investigate the potential role of miR-155 on the development of autoimmune myocarditis. In this study, we revealed that miR-155 expression was highly elevated in heart tissue and CD4+ T cells during EAM. Also, we identified a proliferative and functional imbalance of Th17/Treg in EAM, which is due to a more active development of Th17 cells and an increased resistance of Th17 cells to Treg-mediated suppression. MiR-155 inhibition in EAM resulted in attenuated severity of disease and cardiac injury, reduced Th17 immune response, and decreased dendritic cell (DC) function of secreting Th17-polarizing cytokines. Furthermore, CD4+ T cells from miR-155-inhibited EAM mice exhibited reduced proliferation and IL-17A secretion in response to autoantigen. Finally, we confirmed an indispensable positive role of miR-155 on the differentiation of Th17 cells and the DC function of secreting Th17-polarizing cytokines through in vitro studies. These findings demonstrated that miR-155 adversely promotes EAM by driving a Th17/Treg imbalance in favor of Th17 cells, and anti-miR-155 treatment can significantly reduce the autoimmune response thus to ameliorate EAM, suggesting that miR-155 inhibition could be a promising therapeutic strategy for the treatment of autoimmune myocarditis.
Key message
-
MiR-155 expression was highly elevated in EAM mice.
-
An imbalance of Th17/Treg existed in EAM mice.
-
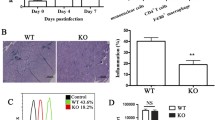
MiR-155 inhibition in EAM attenuated disease severity and cardiac injury.
-
MiR-155 inhibition suppressed Th17 immune response in EAM.
-
MiR-155 inhibition reduced DC function of secreting Th17-polarizing cytokines in EAM.








Similar content being viewed by others
References
Sagar S, Liu PP, Cooper LJ (2012) Myocarditis. Lancet 379:738–747
Caforio ALP, Marcolongo R, Jahns R, Fu M, Felix SB, Iliceto S (2013) Immune-mediated and autoimmune myocarditis: clinical presentation, diagnosis and management. Heart Fail Rev 18:715–732
Root-Bernstein R, Fairweather D (2014) Unresolved issues in theories of autoimmune disease using myocarditis as a framework. J Theor Biol. 10.1016/j.jtbi.2014.11.022*10.1016/j.jtbi.2014.11.022
Muller AM, Fischer A, Katus HA, Kaya Z (2015) Mouse models of autoimmune diseases—autoimmune myocarditis. Curr Pharm Des 21:2498–2512
Wei B, Pei G (2010) microRNAs: critical regulators in Th17 cells and players in diseases. Cell Mol Immunol 7:175–181
Maddur MS, Miossec P, Kaveri SV, Bayry J (2012) Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 181:8–18
Littman DR, Rudensky AY (2010) Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140:845–858
Josefowicz SZ, Lu L, Rudensky AY (2012) Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30:531–564
Chauhan SK, El AJ, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R (2009) Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol 182:1247–1252
Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M (2009) Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum 60:1472–1483
Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C, Gao N, Jia Y, Xu D, Huang FP, Li N, Lu L, Li ZG (2014) Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis. 10.1136/annrheumdis-2013-204228*10.1136/annrheumdis-2013-204228
Ganguly D, Haak S, Sisirak V, Reizis B (2013) The role of dendritic cells in autoimmunity. Nat Rev Immunol 13:566–577
Merad M, Sathe P, Helft J, Miller J, Mortha A (2013) The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol 31:563–604
Mbongue J, Nicholas D, Firek A, Langridge W (2014) The role of dendritic cells in tissue-specific autoimmunity. J Immunol Res 2014:1–17
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Zhu S, Pan W, Qian Y (2013) MicroRNA in immunity and autoimmunity. J Mol Med 91:1039–1050
Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA et al (2007) Requirement of bic/microRNA-155 for normal immune function. Science 316:608–611
Vigorito E, Kohlhaas S, Lu D, Leyland R (2013) miR-155: an ancient regulator of the immune system. Immunol Rev 253:146–157
Blüml S, Bonelli M, Niederreiter B, Puchner A, Mayr G, Hayer S, Koenders MI, van den Berg WB, Smolen J, Redlich K (2011) Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum 63:1281–1288
Min M, Peng L, Yang Y, Guo M, Wang W, Sun G (2014) MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis 20:652–659
Xin Q, Li J, Dang J, Bian X, Shan S, Yuan J, Qian Y, Liu Z, Liu G, Yuan Q et al (2015) miR-155 deficiency ameliorates autoimmune inflammation of systemic lupus erythematosus by targeting S1pr1 in Faslpr/lpr mice. J Immunol 194:5437–5445
Barin JG, Čiháková D (2013) Control of inflammatory heart disease by CD4. ANN NY Acad Sci 1285:80–96
Yuan J, Yu M, Lin QW, Cao AL, Yu X, Dong JH, Wang JP, Zhang JH, Wang M, Guo HP et al (2010) Th17 cells contribute to viral replication in coxsackievirus B3-induced acute viral myocarditis. J Immunol 185:4004–4010
Yamashita T, Iwakura T, Matsui K, Kawaguchi H, Obana M, Hayama A, Maeda M, Izumi Y, Komuro I, Ohsugi Y et al (2011) IL-6-mediated Th17 differentiation through ROR t is essential for the initiation of experimental autoimmune myocarditis. Cardiovasc Res 91:640–648
Huber SA, Feldman AM, Sartini D (2006) Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor- transgenic mice. Circ Res 99:1109–1116
Shi Y, Fukuoka M, Li G, Liu Y, Chen M, Konviser M, Chen X, Opavsky MA, Liu PP (2010) Regulatory T cells protect mice against coxsackievirus-induced myocarditis through the transforming growth factor beta-coxsackie-adenovirus receptor pathway. Circulation 121:2624–2634
Cihakova D, Sharma RB, Fairweather D, Afanasyeva M, Rose NR (2004) Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol Med 102:175–193
Huber SA, Sartini D (2005) Roles of tumor necrosis factor alpha (TNF-alpha) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol 79:2659–2665
Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223:77–92
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408
McMurchy AN, Levings MK (2012) Suppression assays with human T regulatory cells: a technical guide. Eur J Immunol 42:27–34
Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E (2009) Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J Immunol 182:2578–2582
Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K et al (2009) Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity 30:80–91
Linossi EM, Babon JJ, Hilton DJ, Nicholson SE (2013) Suppression of cytokine signaling: the SOCS perspective. Cytokine Growth F R 24:241–248
O’Connell RM, Chaudhuri AA, Rao DS, Baltimore D (2009) Inositol phosphatase SHIP1 is a primary target of miR-155. Proc Natl Acad Sci U S A 106:7113–7118
Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T (2009) MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN). J Biol Chem 284:16334–16342
Lu C, Huang X, Zhang X, Roensch K, Cao Q, Nakayama KI, Blazar BR, Zeng Y, Zhou X (2011) miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood 117:4293–4303
Seddiki N, Brezar V, Ruffin N, Lévy Y, Swaminathan S (2014) Role of miR-155 in the regulation of lymphocyte immune function and disease. Immunology 142:32–38
Tritt M, Sgouroudis E, D’Hennezel E, Albanese A, Piccirillo CA (2008) Functional waning of naturally occurring CD4+ regulatory T-cells contributes to the onset of autoimmune diabetes. Diabetes 57:113–123
Korn T, Anderson AC, Bettelli E, Oukka M (2007) The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol 191:51–60
Krützfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438:685–689
Eriksson U, Ricci R, Hunziker L, Kurrer MO, Oudit GY, Watts TH, Sonderegger I, Bachmaier K, Kopf M, Penninger JM (2003) Dendritic cell–induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nat Med 9:1484–1490
O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D (2010) Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol 10:111–122
Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB et al (2007) Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 13:423–431
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235–238
Stahl HF, Fauti T, Ullrich N, Bopp T, Kubach J, Rust W, Labhart P, Alexiadis V, Becker C, Hafner M et al (2009) miR-155 inhibition sensitizes CD4+ Th cells for TREG mediated suppression. PLoS One 4:e7158
O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D (2008) Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205:585–594
Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL (2010) Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. EUR J IMMUNOL 40: 225-231. doi:10.1002/eji.200939381*10.1002/eji.200939381
Dorsett Y, McBride KM, Jankovic M, Gazumyan A, Thai TH, Robbiani DF, Di Virgilio M, Reina SB, Heidkamp G, Schwickert TA et al (2008) MicroRNA-155 suppresses activation-induced cytidine deaminase-mediated Myc-Igh translocation. Immunity 28:630–638
Yao R, Ma YL, Liang W, Li HH, Ma ZJ, Yu X, Liao YH (2012) MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One 7, e46082. doi:10.1371/journal.pone.0046082*10.1371/journal.pone.0046082
van Hamburg JP, de Bruijn MJ, Ribeiro DAC, van Zwam M, van Meurs M, de Haas E, Boon L, Samsom JN, Hendriks RW (2008) Enforced expression of GATA3 allows differentiation of IL-17-producing cells, but constrains Th17-mediated pathology. Eur J Immunol 38:2573–2586
Lazarevic V, Chen X, Shim JH, Hwang ES, Jang E, Bolm AN, Oukka M, Kuchroo VK, Glimcher LH (2011) T-bet represses T(H)17 differentiation by preventing Runx1-mediated activation of the gene encoding RORgammat. Nat Immunol 12:96–104
Nouraee N, Mowla SJ (2015) miRNA therapeutics in cardiovascular diseases: promises and problems. Frontiers in Genetics 6. 10.3389/fgene.2015.00232*10.3389/fgene.2015.00232.
Acknowledgments
We are grateful to all the members and the facilities of the experimental animal center of the Tongji Medical College for excellent animal care. This project was supported by grant from the National Natural Science Foundation of China (No. 81170205).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Lianhua Yan and Fen Hu contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1359 kb)
Rights and permissions
About this article
Cite this article
Yan, L., Hu, F., Yan, X. et al. Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med 94, 1063–1079 (2016). https://doi.org/10.1007/s00109-016-1414-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1414-3