Abstract
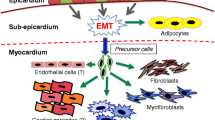
The epithelial to mesenchymal transition (EMT) is a biological process that drives the formation of cells involved both in tissue repair and in pathological conditions, including tissue fibrosis and tumor metastasis by providing cancer cells with stem cell properties. Recent findings suggest that EMT is reactivated in the heart following ischemic injury. Specifically, epicardial EMT might be involved in the formation of cardiac progenitor cells (CPCs) that can differentiate into endothelial cells, smooth muscle cells, and, possibly, cardiomyocytes. The identification of mechanisms and signaling pathways governing EMT-derived CPC generation and differentiation may contribute to the development of a more efficient regenerative approach for adult heart repair. Here, we summarize key literature in the field.



Similar content being viewed by others
References
Sanganalmath SK, Bolli R (2013) Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res 113:810–834
Hay ED (1995) An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 154:8–20
Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
Potenta S, Zeisberg E, Kalluri R (2008) The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer 99:1375–1379
Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R (2007) Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem 282:23337–23347
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A 103:13180–13185
Tsai JH, Yang J (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27:2192–2206
Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA (2004) Snail blocks the cell cycle and confers resistance to cell death. Genes Dev 18:1131–1143
Petersen OW, Nielsen HL, Gudjonsson T, Villadsen R, Rank F, Niebuhr E, Bissell MJ, Ronnov-Jessen L (2003) Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol 162:391–402
Shu X, Pei D (2014) The function and regulation of mesenchymal-to-epithelial transition in somatic cell reprogramming. Curr Opin Genet Dev 28C:32–37
Baum B, Settleman J, Quinlan MP (2008) Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol 19:294–308
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15:178–196
Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC (2003) TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol 284:F243–F252
Gressner AM, Weiskirchen R, Breitkopf K, Dooley S (2002) Roles of TGF-beta in hepatic fibrosis. Front Biosci 7:d793–d807
Willis BC, Borok Z (2007) TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293:L525–L534
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB et al (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961
Hudson LG, Newkirk KM, Chandler HL, Choi C, Fossey SL, Parent AE, Kusewitt DF (2009) Cutaneous wound reepithelialization is compromised in mice lacking functional Slug (Snai2). J Dermatol Sci 56:19–26
Kusewitt DF, Choi C, Newkirk KM, Leroy P, Li Y, Chavez MG, Hudson LG (2009) Slug/Snai2 is a downstream mediator of epidermal growth factor receptor-stimulated reepithelialization. J Investig Dermatol 129:491–495
Aisagbonhi O, Rai M, Ryzhov S, Atria N, Feoktistov I, Hatzopoulos AK (2011) Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis Model Mech 4:469–483
Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, Kulesz-Martin M, Bottinger E, Wang XJ (2008) Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest 118:2722–2732
Kotiyal S, Bhattacharya S (2014) Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun 453:112–116
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 3, e2888
Caja L, Bertran E, Campbell J, Fausto N, Fabregat I (2011) The transforming growth factor-beta (TGF-beta) mediates acquisition of a mesenchymal stem cell-like phenotype in human liver cells. J Cell Physiol 226:1214–1223
Gonzalez DM, Medici D (2014) Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal 7:re8
Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT (2007) Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med 13:970–974
Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H et al (2009) Evidence for cardiomyocyte renewal in humans. Science 324:98–102
Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT (2013) Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493:433–436
Garbern JC, Lee RT (2013) Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 12:689–698
Porrello ER, Olson EN (2014) A neonatal blueprint for cardiac regeneration. Stem Cell Res 13(3 Pt B):556–570
Bergmann O, Jovinge S (2014) Cardiac regeneration in vivo: mending the heart from within? Stem Cell Res 13(3 Pt B):523–531
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science 331:1078–1080
Andersen DC, Ganesalingam S, Jensen CH, Sheikh SP (2014) Do neonatal mouse hearts regenerate following heart apex resection? Stem Cell Rep 2:406–413
Andersen DC, Jensen CH, Sheikh SP (2014) Response to Sadek et al. and Kotlikoff et al. Stem Cell Rep 3:3–4
Sadek HA, Martin JF, Takeuchi JK, Leor J, Nei Y, Giacca M, Lee RT (2014) Multi-investigator letter on reproducibility of neonatal heart regeneration following apical resection. Stem Cell Rep 3:1
Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R (2014) Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A 111:8850–8855
Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K et al (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114:763–776
Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F et al (2005) Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 102:8966–8971
Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N et al (2007) Human cardiac stem cells. Proc Natl Acad Sci U S A 104:14068–14073
Chong JJ, Forte E, Harvey RP (2014) Developmental origins and lineage descendants of endogenous adult cardiac progenitor cells. Stem Cell Res 13:592–614
van Berlo JH, Molkentin JD (2014) An emerging consensus on cardiac regeneration. Nat Med 20:1386–1393
Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N et al (2009) Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A 106:17169–17174
Bearzi C, Leri A, Lo Monaco F, Rota M, Gonzalez A, Hosoda T, Pepe M, Qanud K, Ojaimi C, Bardelli S et al (2009) Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci U S A 106:15885–15890
Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM et al (2009) c-Kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A 106:1808–1813
Zaruba MM, Soonpaa M, Reuter S, Field LJ (2010) Cardiomyogenic potential of c-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation 121:1992–2000
Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK et al (2012) c-Kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci U S A 109:13380–13385
Ellison GM, Torella D, Dellegrottaglie S, Perez-Martinez C, Perez de Prado A, Vicinanza C, Purushothaman S, Galuppo V, Iaconetti C, Waring CD et al (2011) Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol 58:977–986
Yaniz-Galende E, Chen J, Chemaly E, Liang L, Hulot JS, McCollum L, Arias T, Fuster V, Zsebo KM, Hajjar RJ (2012) Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells. Circ Res 111:1434–1445
Zhang L, Chen B, Zhao Y, Dubielecka PM, Wei L, Qin GJ, Chin YE, Wang Y, Zhao TC (2012) Inhibition of histone deacetylase-induced myocardial repair is mediated by c-kit in infarcted hearts. J Biol Chem 287:39338–39348
Ellison GM, Vicinanza C, Smith AJ, Aquila I, Leone A, Waring CD, Henning BJ, Stirparo GG, Papait R, Scarfo M et al (2013) Adult c-kit(pos) cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154:827–842
van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD (2014) c-Kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509:337–341
Molkentin JD, Houser SR (2013) Are resident c-Kit+ cardiac stem cells really all that are needed to mend a broken heart? Circ Res 113:1037–1039
Torella D, Ellison GM, Nadal-Ginard B (2014) Adult c-kit(pos) cardiac stem cells fulfill Koch’s postulates as causal agents for cardiac regeneration. Circ Res 114:e24–e26
Molkentin JD, Houser SR (2014) Response to Torella et al. Circ Res 114, e27
Nadal-Ginard B, Ellison GM, Torella D (2014) Absence of evidence is not evidence of absence: pitfalls of Cre Knock-Ins in the c-Kit locus. Circ Res 115:415–418
Molkentin JD (2014) Letter by Molkentin regarding article, “the absence of evidence is not evidence of absence: the pitfalls of Cre Knock-Ins in the c-Kit locus”. Circ Res 115:e21–e23
Nadal-Ginard B, Ellison GM, Torella D (2014) Response to Molkentin’s letter to the editor regarding article, “the absence of evidence is not evidence of absence: the pitfalls of Cre Knock-Ins in the c-Kit locus”. Circ Res 115:e38–e39
Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS et al (2011) Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest 121:1894–1904
van Wijk B, Gunst QD, Moorman AF, van den Hoff MJ (2012) Cardiac regeneration from activated epicardium. PLoS One 7, e44692
Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF et al (2011) De novo cardiomyocytes from within the activated adult heart after injury. Nature 474:640–644
Castaldo C, Di Meglio F, Nurzynska D, Romano G, Maiello C, Bancone C, Muller P, Bohm M, Cotrufo M, Montagnani S (2008) CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells 26:1723–1731
Di Meglio F, Castaldo C, Nurzynska D, Romano V, Miraglia R, Bancone C, Langella G, Vosa C, Montagnani S (2010) Epithelial-mesenchymal transition of epicardial mesothelium is a source of cardiac CD117-positive stem cells in adult human heart. J Mol Cell Cardiol 49:719–727
Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M et al (2007) Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res 101:1255–1265
Limana F, Bertolami C, Mangoni A, Di Carlo A, Avitabile D, Mocini D, Iannelli P, De Mori R, Marchetti C, Pozzoli O et al (2010) Myocardial infarction induces embryonic reprogramming of epicardial c-kit(+) cells: role of the pericardial fluid. J Mol Cell Cardiol 48:609–618
Hinz B (2007) Formation and function of the myofibroblast during tissue repair. J Investig Dermatol 127:526–537
Snider P, Olaopa M, Firulli AB, Conway SJ (2007) Cardiovascular development and the colonizing cardiac neural crest lineage. ScientificWorldJournal 7:1090–1113
Chua KN, Poon KL, Lim J, Sim WJ, Huang RY, Thiery JP (2011) Target cell movement in tumor and cardiovascular diseases based on the epithelial-mesenchymal transition concept. Adv Drug Deliv Rev 63:558–567
Ratajska A, Czarnowska E, Ciszek B (2008) Embryonic development of the proepicardium and coronary vessels. Int J Dev Biol 52:229–236
Manner J, Perez-Pomares JM, Macias D, Munoz-Chapuli R (2001) The origin, formation and developmental significance of the epicardium: a review. Cells Tissues Organs 169:89–103
Perez-Pomares JM, de la Pompa JL (2011) Signaling during epicardium and coronary vessel development. Circ Res 109:1429–1442
Reese DE, Mikawa T, Bader DM (2002) Development of the coronary vessel system. Circ Res 91:761–768
Wessels A, Perez-Pomares JM (2004) The epicardium and epicardially derived cells (EPDCs) as cardiac stem cells. Anat Rec A: Discov Mol Cell Evol Biol 276:43–57
Lie-Venema H, van den Akker NM, Bax NA, Winter EM, Maas S, Kekarainen T, Hoeben RC, deRuiter MC, Poelmann RE, Gittenberger-de Groot AC (2007) Origin, fate, and function of epicardium-derived cells (EPDCs) in normal and abnormal cardiac development. ScientificWorldJournal 7:1777–1798
Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X et al (2008) A myocardial lineage derives from Tbx18 epicardial cells. Nature 454:104–108
Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR et al (2008) Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 454:109–113
Smith CL, Baek ST, Sung CY, Tallquist MD (2011) Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res 108:e15–e26
Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM (2005) The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 132:5317–5328
Tian X, Pu WT, Zhou B (2015) Cellular origin and developmental program of coronary angiogenesis. Circ Res 116:515–530
Red-Horse K, Ueno H, Weissman IL, Krasnow MA (2010) Coronary arteries form by developmental reprogramming of venous cells. Nature 464:549–553
Wu B, Zhang Z, Lui W, Chen X, Wang Y, Chamberlain AA, Moreno-Rodriguez RA, Markwald RR, O’Rourke BP, Sharp DJ et al (2012) Endocardial cells form the coronary arteries by angiogenesis through myocardial-endocardial VEGF signaling. Cell 151:1083–1096
Poelmann RE, Gittenberger-de Groot AC, Mentink MM, Bokenkamp R, Hogers B (1993) Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ Res 73:559–568
Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Zhang Z et al (2013) Subepicardial endothelial cells invade the embryonic ventricle wall to form coronary arteries. Cell Res 23:1075–1090
Tian X, Hu T, Zhang H, He L, Huang X, Liu Q, Yu W, He L, Yang Z, Yan Y et al (2014) Vessel formation. De novo formation of a distinct coronary vascular population in neonatal heart. Science 345:90–94
Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ (2006) BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol 295:507–522
van Wijk B, van den Berg G, Abu-Issa R, Barnett P, van der Velden S, Schmidt M, Ruijter JM, Kirby ML, Moorman AF, van den Hoff MJ (2009) Epicardium and myocardium separate from a common precursor pool by crosstalk between bone morphogenetic protein- and fibroblast growth factor-signaling pathways. Circ Res 105:431–441
Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A (2009) Tbx18 and the fate of epicardial progenitors. Nature 458:E8–E9
Rudat C, Kispert A (2012) Wt1 and epicardial fate mapping. Circ Res 111:165–169
Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A et al (2011) Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell 9:527–540
Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD (2010) Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464:601–605
Lien CL, Harrison MR, Tuan TL, Starnes VA (2012) Heart repair and regeneration: recent insights from zebrafish studies. Wound Repair Regen 20:638–646
Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD (2006) A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127:607–619
Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD (2011) Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev Cell 20:397–404
Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, Shimada H, Handin RI, Chao MY, Tuan TL et al (2010) PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci U S A 107:17206–17210
Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD (2011) Tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 138:2895–2902
Zhou B, Honor LB, Ma Q, Oh JH, Lin RZ, Melero-Martin JM, von Gise A, Zhou P, Hu T, He L et al (2012) Thymosin beta 4 treatment after myocardial infarction does not reprogram epicardial cells into cardiomyocytes. J Mol Cell Cardiol 52:43–47
Bollini S, Vieira JM, Howard S, Dube KN, Balmer GM, Smart N, Riley PR (2014) Re-activated adult epicardial progenitor cells are a heterogeneous population molecularly distinct from their embryonic counterparts. Stem Cells Dev 23:1719–1730
Asli NS, Xaymardan M, Harvey RP (2014) Epicardial origin of resident mesenchymal stem cells in the adult mammalian heart. J Dev Biol 2:117–137
Zakharova L, Nural-Guvener H, Gaballa MA (2012) Cardiac explant-derived cells are regulated by Notch-modulated mesenchymal transition. PLoS One 7, e37800
Forte E, Miraldi F, Chimenti I, Angelini F, Zeuner A, Giacomello A, Mercola M, Messina E (2012) TGFbeta-dependent epithelial-to-mesenchymal transition is required to generate cardiospheres from human adult heart biopsies. Stem Cells Dev 21:3081–3090
Bax NA, van Oorschot AA, Maas S, Braun J, van Tuyn J, de Vries AA, Groot AC, Goumans MJ (2011) In vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFbeta-signaling and WT1. Basic Res Cardiol 106:829–847
Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P et al (2010) Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Genet 42:89–93
Takeichi M, Nimura K, Mori M, Nakagami H, Kaneda Y (2013) The transcription factors Tbx18 and Wt1 control the epicardial epithelial-mesenchymal transition through bi-directional regulation of Slug in murine primary epicardial cells. PLoS One 8, e57829
Di Meglio F, Castaldo C, Nurzynska D, Romano V, Miraglia R, Montagnani S (2010) Epicardial cells are missing from the surface of hearts with ischemic cardiomyopathy: a useful clue about the self-renewal potential of the adult human heart? Int J Cardiol 145:e44–e46
Xiang FL, Liu Y, Lu X, Jones DL, Feng Q (2014) Cardiac-specific overexpression of human stem cell factor promotes epicardial activation and arteriogenesis after myocardial infarction. Circ Heart Fail 7:831–842
Grieskamp T, Rudat C, Ludtke TH, Norden J, Kispert A (2011) Notch signaling regulates smooth muscle differentiation of epicardium-derived cells. Circ Res 108:813–823
Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW (2011) A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res 108:51–59
Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y (2007) Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene 26:2781–2790
Bock-Marquette I, Shrivastava S, Pipes GC, Thatcher JE, Blystone A, Shelton JM, Galindo CL, Melegh B, Srivastava D, Olson EN et al (2009) Thymosin beta4 mediated PKC activation is essential to initiate the embryonic coronary developmental program and epicardial progenitor cell activation in adult mice in vivo. J Mol Cell Cardiol 46:728–738
Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, Riley PR (2007) Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 445:177–182
Shin SH, Lee S, Bae JS, Jee JG, Cha HJ, Lee YM (2014) Thymosin beta4 regulates cardiac valve formation via endothelial-mesenchymal transformation in zebrafish embryos. Mol Cells 37:330–336
Deb A (2014) Cell-cell interaction in the heart via Wnt/beta-catenin pathway after cardiac injury. Cardiovasc Res 102:214–223
Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A et al (2012) Wnt1/beta catenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J 31:429–442
Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D (2007) Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A 104:10894–10899
Oikonomopoulos A, Sereti KI, Conyers F, Bauer M, Liao A, Guan J, Crapps D, Han JK, Dong H, Bayomy AF et al (2011) Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ Res 109:1363–1374
Yang L, Jung Y, Omenetti A, Witek RP, Choi S, Vandongen HM, Huang J, Alpini GD, Diehl AM (2008) Fate-mapping evidence that hepatic stellate cells are epithelial progenitors in adult mouse livers. Stem Cells 26:2104–2113
Kocabas F, Mahmoud AI, Sosic D, Porrello ER, Chen R, Garcia JA, DeBerardinis RJ, Sadek HA (2012) The hypoxic epicardial and subepicardial microenvironment. J Cardiovasc Transl Res 5:654–665
Winter EM, Gittenberger-de Groot AC (2007) Epicardium-derived cells in cardiogenesis and cardiac regeneration. Cell Mol Life Sci 64:692–703
Winter EM, van Oorschot AA, Hogers B, van der Graaf LM, Doevendans PA, Poelmann RE, Atsma DE, Gittenberger-de Groot AC, Goumans MJ (2009) A new direction for cardiac regeneration therapy: application of synergistically acting epicardium-derived cells and cardiomyocyte progenitor cells. Circ Heart Fail 2:643–653
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Simons M, Raposo G (2009) Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581
Gupta SK, Bang C, Thum T (2010) Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet 3:484–488
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta 1841:108–120
Cosme J, Liu PP, Gramolini AO (2013) The cardiovascular exosome: current perspectives and potential. Proteomics 13:1654–1659
Sahoo S, Losordo DW (2014) Exosomes and cardiac repair after myocardial infarction. Circ Res 114:333–344
Waldenstrom A, Ronquist G (2014) Role of exosomes in myocardial remodeling. Circ Res 114:315–324
Yellon DM, Davidson SM (2014) Exosomes: nanoparticles involved in cardioprotection? Circ Res 114:325–332
Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y (2013) Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431:566–571
Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA (2010) Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 14:1064–1070
Zhang J, Zhang H, Liu J, Tu X, Zang Y, Zhu J, Chen J, Dong L, Zhang J (2012) miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun 417:1100–1105
Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB (2012) miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther 11:1166–1173
Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH et al (2011) p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13:317–323
Khew-Goodall Y, Goodall GJ (2010) Myc-modulated miR-9 makes more metastases. Nat Cell Biol 12:209–211
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10:593–601
Lamouille S, Subramanyam D, Blelloch R, Derynck R (2013) Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol 25:200–207
Bronnum H, Andersen DC, Schneider M, Sandberg MB, Eskildsen T, Nielsen SB, Kalluri R, Sheikh SP (2013) miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PLoS One 8:e56280
Krichevsky AM, Gabriely G (2009) miR-21: a small multi-faceted RNA. J Cell Mol Med 13:39–53
Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A et al (2014) Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 124:2136–2146
Rupp H, Rupp TP, Alter P, Jung N, Pankuweit S, Maisch B (2010) Intrapericardial procedures for cardiac regeneration by stem cells: need for minimal invasive access (AttachLifter) to the normal pericardial cavity. Herz 35:458–465
Acknowledgments
We apologize to the many researchers whose work was not cited in this review. We thank Dr. Giuseppe Aleo for the English editing of the manuscript. The authors are supported by Ministero della Salute (Italian Ministry of Public Health).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Germani, A., Foglio, E., Capogrossi, M.C. et al. Generation of cardiac progenitor cells through epicardial to mesenchymal transition. J Mol Med 93, 735–748 (2015). https://doi.org/10.1007/s00109-015-1290-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1290-2