Abstract
Proliferation and migration disorders of vascular smooth muscle cells (VSMCs) contribute to the pathogenesis of proliferative cardiovascular diseases. Although, over the past two decades, a large panel of drugs has been developed for targeting VSMC proliferation, cardiovascular disease remains the leading cause of death worldwide. Thus, there is a compelling need to identify novel signaling pathways and molecules controlling VSMC proliferation and migration, to provide not only mechanistic insights but also safe and effective therapies for the treatment of cardiovascular diseases. Our recent studies have demonstrated that p55γ, a regulatory subunit of phosphoinositide 3-kinase, functions as an endogenous brake on VSMC proliferation. Here, we demonstrate that the small peptide N24, the first 24 amino acids of the NH2 terminus of p55γ, is a functional mimetic which negatively regulates VSMC proliferation and migration. Specifically, luminal delivery of adenovirus expressing N24 or local administration of Tat transactivator protein (TAT)-tagged N24 by pluronic gel alleviates neointimal formation following balloon injury in rat carotid arteries. Enforced expression of N24 suppresses the proliferation and migration of VSMCs induced by serum- or platelet-derived growth factor-BB. Mechanistically, N24 induces cell cycle arrest via activating the p53–p21 signal pathway, without triggering cell death. N24 interacts with and stabilizes p53 by blocking its ubiquitin-dependent degradation, subsequently promotes p21 transcription, and arrests cell cycle progression. Indeed, knockdown of p21 or p53 abrogates the N24-mediated cell growth arrest. Thus, N24 is a p55γ mimetic inhibiting VSMC proliferation as well as migration, thereby conferring important therapeutic implications for anti-proliferative treatment.
Key message
-
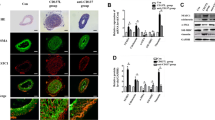
N24 attenuates balloon injury-induced neointimal formation.
-
Overexpression of N24 inhibits cultured VSMC proliferation and migration.
-
Overexpression of N24 arrests the cell cycle at S phase.
-
N24 interacts with and stabilizes p53 resulting in growth suppression.






Similar content being viewed by others
Abbreviations
- VSMC:
-
Vascular smooth muscle cell
- PI3K:
-
Phosphoinositide 3-kinase
- PDGF:
-
Platelet-derived growth factor
- CDK:
-
Cyclin-dependent kinase
- TAT:
-
Tat transactivator protein
- FACS:
-
Fluorescence-activated cell sorting
- PCNA:
-
Proliferating cell nuclear antigen
- IP:
-
Immunoprecipitation
- Ub:
-
Ubiquitin
References
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801
Dzau VJ, Braun-Dullaeus RC, Sedding DG (2002) Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med 8:1249–1256
Heldin CH, Westermark B (1999) Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev 79:1283–1316
Majesky MW, Lindner V, Twardzik DR, Schwartz SM, Reidy MA (1991) Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest 88:904–910
Millar JA, Harris EL, Cassie NJ (1990) Mitogenesis in cultured vascular smooth muscle cells from two rat models of hypertension in response to fetal calf serum and angiotensin II. J Cardiovasc Pharmacol 16:S14–S16
Braun-Dullaeus RC, Mann MJ, Dzau VJ (1998) Cell cycle progression: new therapeutic target for vascular proliferative disease. Circulation 98(1):82–89
Murray AW (1987) Cell cycle control. A cycle is a cycle is a cycle. Nature 327:14–15
Sherr CJ, Roberts JM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Gene Dev 9:1149–1163
el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825
Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G et al (2002) A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 346:1773–1780
Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J et al (2004) A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 350:221–231
Soroceanu L, Kharbanda S, Chen R, Soriano RH, Aldape K, Misra A, Zha J, Forrest WF, Nigro JM, Modrusan Z et al (2007) Identification of IGF2 signaling through phosphoinositide-3-kinase regulatory subunit 3 as a growth-promoting axis in glioblastoma. Proc Natl Acad Sci U S A 104:3466–3471
Zhang L, Huang J, Yang N, Greshock J, Liang S, Hasegawa K, Giannakakis A, Poulos N, O’Brien-Jenkins A, Katsaros D et al (2007) Integrative genomic analysis of phosphatidylinositol 3′-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin Cancer Res 13:5314–5321
Li G, Xie N, Yao Y, Zhang Y, Guo J, Feng Y, Lv F, Xiao RP, Cao CM (2014) Identification of PI3K regulatory subunit p55gamma as a novel inhibitor of vascular smooth muscle cell proliferation and neointimal formation. Cardiovasc Res 105:75–85
Pons S, Asano T, Glasheen E, Miralpeix M, Zhang Y, Fisher TL, Myers MG Jr, Sun XJ, White MF (1995) The structure and function of p55PIK reveal a new regulatory subunit for phosphatidylinositol 3-kinase. Mol Cell Biol 15:4453–4465
Xia X, Cheng A, Akinmade D, Hamburger AW (2003) The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol Cell Biol 23:1717–1725
Wang G, Deng Y, Cao X, Lai S, Tong Y, Luo X, Feng Y, Xia X, Gong J, Hu J (2012) Blocking p55PIK signaling inhibits proliferation and induces differentiation of leukemia cells. Cell Death Differ 19:1870–1879
Hu J, Xia X, Cheng A, Wang G, Luo X, Reed MF, Fojo T, Oetting A, Gong J, Yen PM (2008) A peptide inhibitor derived from p55PIK phosphatidylinositol 3-kinase regulatory subunit: a novel cancer therapy. Mol Cancer Ther 7:3719–3728
Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu X, Wen S, Xiao RP et al (2004) Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol 6:872–883
Cequier A, Bonan R, Crepeau J, Cote G, De Guise P, Joly P, Lesperance J, Waters DD (1988) Restenosis and progression of coronary atherosclerosis after coronary angioplasty. J Am Coll Cardiol 12:49–55
Li Q, Li G, Lan X, Zheng M, Chen KH, Cao CM, Xiao RP (2010) Receptor interacting protein 3 suppresses vascular smooth muscle cell growth by inhibition of the phosphoinositide 3-kinase-Akt axis. J Biol Chem 285:9535–9544
Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A (2006) Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharmacol 9:339–358
Haupt Y, Maya R, Kazaz A, Oren M (1997) Mdm2 promotes the rapid degradation of p53. Nature 387:296–299
Hanahan D, Weinberg Robert A (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Gupta GP, Massagué J (2006) Cancer metastasis: building a framework. Cell 127:679–695
Brosh R, Rotter V (2009) When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer 9:701–713
Muller PAJ, Vousden KH, Norman JC (2011) p53 and its mutants in tumor cell migration and invasion. J Cell Biol 192:209–218
Xia X, Serrero G (1999) Multiple forms of p55PIK, a regulatory subunit of phosphoinositide 3-kinase, are generated by alternative initiation of translation. Biochem J 341:831–837
Fukui R, Shibata N, Kohbayashi E, Amakawa M, Furutama D, Hoshiga M, Negoro N, Nakakouji T, Ii M, Ishihara T et al (1997) Inhibition of smooth muscle cell migration by the p21 cyclin-dependent kinase inhibitor (Cip1). Atherosclerosis 132:53–59
Witzenbichler B, Kureishi Y, Luo Z, Le Roux A, Branellec D, Walsh K (1999) Regulation of smooth muscle cell migration and integrin expression by the Gax transcription factor. J Clin Invest 104:1469–1480
George SJ, Angelini GD, Capogrossi MC, Baker AH (2001) Wild-type p53 gene transfer inhibits neointima formation in human saphenous vein by modulation of smooth muscle cell migration and induction of apoptosis. Gene Ther 8:668–676
Inukai K, Funaki M, Nawano M, Katagiri H, Ogihara T, Anai M, Onishi Y, Sakoda H, Ono H, Fukushima Y et al (2000) The N-terminal 34 residues of the 55 kDa regulatory subunits of phosphoinositide 3-kinase interact with tubulin. Biochem J 346:483–489
Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR (2006) Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med 12:1075–1080
Roiron C, Sanchez P, Bouzamondo A, Lechat P, Montalescot G (2006) Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart 92:641–649
Simsek C, Magro M, Boersma E, Onuma Y, Nauta ST, Gaspersz MP, van der Giessen WJ, van Domburg RT, Serruys PW (2010) The unrestricted use of sirolimus- and paclitaxel-eluting stents results in better clinical outcomes during 6-year follow-up than bare-metal stents: an analysis of the RESEARCH (Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital) and T-SEARCH (Taxus-Stent Evaluated At Rotterdam Cardiology Hospital) registries. JACC Cardiovasc Interv 3:1051–1058
Acknowledgments
We thank Dr. X. Xia for the generous gift of Ad-N24 and TAT-N24, and J Zhang and N Hou for their excellent technical supports. This work was supported by the National Basic Research Program of China (2012CB944500, 2012CB518000, and 2013CB531200), the National Natural Science Foundation of China (9143910034, 81370233, 81370234, 81130073, 81170100 and 31221002), and the Beijing Municipal Scientific and Technology Commission (2007B005).
Conflict of interest
All authors confirm that there is no conflict of interest associated with this publication.
Authors contributions
Jiaojiao Guo conducted key in vitro experiments. Ning Xie generated the in vivo experiments. Chun-Mei Cao and Rui-Ping Xiao designed the study and wrote the manuscript. Geng Li, Yan Zhang, Fengxiang Lv, Sile Guo, and Yuanqing Feng performed the experiments.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 313 kb)
Rights and permissions
About this article
Cite this article
Guo, J., Xie, N., Li, G. et al. p55γ functional mimetic peptide N24 blocks vascular proliferative disorders. J Mol Med 93, 1107–1118 (2015). https://doi.org/10.1007/s00109-015-1287-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1287-x