Abstract
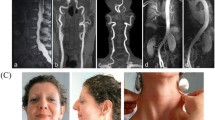
Excessive activation of the transforming growth factor beta signaling pathway and disorganized cellular skeleton caused by genetic mutations are known to be responsible for the inherited thoracic aortic aneurysms and dissections (TAAD), a life-threatening vascular disease. To investigate the genotype–phenotype correlation, we screened genetic mutations of fibrillin-1 (FBN1), transforming growth factor-β receptor-1 (TGFBR1) and transforming growth factor-β receptor-2 (TGFBR2) for TAAD in 7 affected families and 22 sporadic patients. Of 19 potential mutations identified in FBN1, 11 appeared novel while the others were recurrent. Two mutations were detected in TGFBR2. Eight patients carried no mutation in either of these genes. Characterization of FBN1 c.5917+6T>C in transfected HEK293 cells demonstrated that it caused skipping of exon 47, leading to the loss of the 33th calcium binding epidermal growth factor-like domain associated with Marfan syndrome. Compared with exon 46, skipping of 47 did not cause patients ectopia lentis in all carriers. To correlate genotypes with phenotypes in different human ancestries, we reviewed the published mutational studies on FBN1 and found that the probability of cardiovascular defects were significantly increased in Chinese patients with premature termination codon or splicing mutations than those with missense mutations (91.7 % vs 54.2 %, P = 0.0307) or with noncysteine-involved point mutations than those with cysteine-involved mutations (88.9 % vs 33.3 %, P = 0.0131). Thus, we conclude that exon 47 skipping of FBN1 leads preferentially to cardiovascular defects and human ancestries influence genotype–phenotype correlation in TAAD.




Similar content being viewed by others
References
Mizuguchi T, Matsumoto N (2007) Recent progress in genetics of Marfan syndrome and Marfan-associated disorders. J Hum Genet 52:1–12
Lemaire SA, Russell L (2011) Epidemiology of thoracic aortic dissection. Nat Rev Cardiol 8(2):103–113
Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM et al (1991) Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352:337–339
Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N et al (2005) A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37:275–281
Kainulainen K, Pulkkinen L, Savolainen A, Kaitila I, Peltonen L (1990) Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med 323:935–939
Pope FM, Nicholls AC, Narcisi P, Temple A, Chia Y, Fryer P, De Paepe A, De Groote WP, McEwan JR, Compston DA et al (1988) Type III collagen mutations in Ehlers Danlos syndrome type IV and other related disorders. Clin Exp Dermatol 13:285–302
Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S et al (2006) Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 355:788–798
Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ et al (2005) Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation 112:513–520
Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E et al (2007) Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 39:1488–1493
Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F et al (2006) Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 38:343–349
Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos-Cortez RL, Leal SM et al (2011) Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res 109:680–686
Lemaire SA, McDonald ML, Guo DC, Russell L, 3rd Miller CC, Johnson RJ, Bekheirnia MR, Franco LM, Nguyen M, Pyeritz RE et al (2011) Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat Genet 43:996–1000. doi:10.1038/ng.934
Kaartinen V, Warburton D (2003) Fibrillin controls TGF-beta activation. Nat Genet 33:331–332
Korkko J, Kaitila I, Lonnqvist L, Peltonen L, Ala-Kokko L (2002) Sensitivity of conformation sensitive gel electrophoresis in detecting mutations in Marfan syndrome and related conditions. J Med Genet 39:34–41
Faivre L, Collod-Beroud G, Loeys BL, Child A, Binquet C, Gautier E, Callewaert B, Arbustini E, Mayer K, Arslan-Kirchner M et al (2007) Effect of mutation type and location on clinical outcome in 1,013 probands with Marfan syndrome or related phenotypes and FBN1 mutations: an international study. Am J Hum Genet 81:454–466
Rommel K, Karck M, Haverich A, von Kodolitsch Y, Rybczynski M, Muller G, Singh KK, Schmidtke J, Arslan-Kirchner M (2005) Identification of 29 novel and nine recurrent fibrillin-1 (FBN1) mutations and genotype-phenotype correlations in 76 patients with Marfan syndrome. Hum Mutat 26:529–539
Tian XL, Wang QK (2006) Generation of transgenic mice for cardiovascular research. Meth Mol Med 129:69–81
Attanasio M, Lapini I, Evangelisti L, Lucarini L, Giusti B, Porciani M, Fattori R, Anichini C, Abbate R, Gensini G et al (2008) FBN1 mutation screening of patients with Marfan syndrome and related disorders: detection of 46 novel FBN1 mutations. Clin Genet 74:39–46
Comeglio P, Johnson P, Arno G, Brice G, Evans A, Aragon-Martin J, da Silva FP, Kiotsekoglou A, Child A (2007) The importance of mutation detection in Marfan syndrome and Marfan-related disorders: report of 193 FBN1 mutations. Hum Mutat 28:928
Hung CC, Lin SY, Lee CN, Cheng HY, Lin SP, Chen MR, Chen CP, Chang CH, Lin CY, Yu CC et al (2009) Mutation spectrum of the fibrillin-1 (FBN1) gene in Taiwanese patients with Marfan syndrome. Ann Hum Genet 73:559–567
Soylen B, Singh KK, Abuzainin A, Rommel K, Becker H, Arslan-Kirchner M, Schmidtke J (2009) Prevalence of dural ectasia in 63 gene-mutation-positive patients with features of Marfan syndrome type 1 and Loeys-Dietz syndrome and report of 22 novel FBN1 mutations. Clin Genet 75:265–270
Zhao L, Liang T, Xu J, Lin H, Li D, Qi Y (2009) Two novel FBN1 mutations associated with ectopia lentis and marfanoid habitus in two Chinese families. Mol Vis 15:826–832
Jin C, Yao K, Sun Z, Wu R (2008) Correlation of the recurrent FBN1 mutation (c.364C>T) with a unique phenotype in a Chinese patient with Marfan syndrome. Jpn J Ophthalmol 52:497–499
Qin Y, Yan J, Simpson JL, Gu HF, Wang LC, Chen ZJ (2007) Novel non-synonymous mutation in the transforming growth factor beta binding protein-like (TB) domain of the fibrillin-1 (FBN1) gene in a Han Chinese family with Marfan syndrome (MFS). Neuro Endocrinol Lett 28:629–632
Jin C, Yao K, Jiang J, Tang X, Shentu X, Wu R (2007) Novel FBN1 mutations associated with predominant ectopia lentis and marfanoid habitus in Chinese patients. Mol Vis 13:1280–1284
Wang B, Hu D, Xia J, Li Q, Yang J, Lu G (2003) FBN1 mutation in Chinese patients with Marfan syndrome and its gene diagnosis using haplotype linkage analysis. Chin Med J (Engl) 116:1043–1046
Lo IF, Wong RM, Lam FW, Tong TM, Lam ST (2001) Missense mutations of the fibrillin-1 gene in two Chinese patients with severe Marfan syndrome. Chin Med J (Engl) 114:473–476
Li D, Yu J, Gu F, Pang X, Ma X, Li R, Liu N (2008) The roles of two novel FBN1 gene mutations in the genotype–phenotype correlations of Marfan syndrome and ectopia lentis patients with marfanoid habitus. Genet Test 12:325–330
Sui RF, Wei HB, Zhao JL, Hu SY, Wang B, Huang SZ, Dong M (2004) Novel mutation of fibrillin 1 gene cause ectopia lentis in a Chinese family. Zhonghua Yan Ke Za Zhi 40:828–831
Yu R, Lai Z, Zhou W, Ti DD, Zhang XN (2006) Recurrent FBN1 mutation (R62C) in a Chinese family with isolated ectopia lentis. Am J Ophthalmol 141:1136–1138
van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H et al (2011) Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 43:121–126. doi:10.1038/ng.744
Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD et al (2011) Noncanonical TGFbeta signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332:358–361
Pereira L, Dalessio M, Ramirez F, Lynch JR, Sykes B, Pangilinan T, Bonadio J (1993) Genomic organization of the sequence coding for fibrillin, the defective gene-product in Marfan-syndrome. Hum Mol Genet 2:961–968
Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM (2007) Fibrillin-1 regulates the bioavailability of TGF beta 1. J Cell Biol 176:355–367
Whiteman P, Willis AC, Warner A, Brown J, Redfield C, Handford PA (2007) Cellular and molecular studies of Marfan syndrome mutations identify co-operative protein folding in the cbEGF12-13 region of fibrillin-1. Hum Mol Genet 16:907–918
Acknowledgments
We thank all the patients and their families for their participation. This study was supported by grants from the National Basic Research Program of the Chinese Ministry of Science and Technology (973 grant no. 2007CB512100) and Key Program from the National Natural Science Foundation of China (NSFC grant no. 30730047).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
W.-J. Wang and P. Han contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 504 kb)
Rights and permissions
About this article
Cite this article
Wang, WJ., Han, P., Zheng, J. et al. Exon 47 skipping of fibrillin-1 leads preferentially to cardiovascular defects in patients with thoracic aortic aneurysms and dissections. J Mol Med 91, 37–47 (2013). https://doi.org/10.1007/s00109-012-0931-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0931-y