Abstract
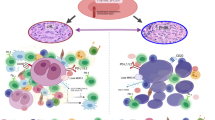
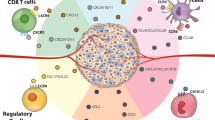
The interaction between lymphoid tumor cells and their tissue microenvironment is thought to promote dissemination and progression of lymphoma. Those type of interactions consists of at least three cornerstones, among them mesenchymal- or bone marrow-derived stromal cells, cells of the innate or adaptive immune response, and the lymphoma cells themselves. The molecular pathways of crosstalk between the lymphoma cells and their nursing stroma are not well understood and their dissection is challenging because of (1) the complexity of stroma cell subpopulations, (2) kinetic and developmental transitions/switches of stroma composition, and (3) inherent technical difficulties to isolate and analyze defined stroma cell subsets. However, recent studies of bone marrow stroma interaction with leukemia or lymphoma cells have revealed therapeutic targets involved in regulating tumor cell mobilization. Release of tumor cells from their supportive niches could be effectuated by inhibition of homing and retention signals. The present review focuses on the effects of homing receptors and cytokines attributed to lymphoid tissue formation in tumor–stroma interactions within secondary lymphoid tissues. We discuss possible cellular and molecular mechanisms of lymphoma–stroma crosstalk and highlight novel therapeutic strategies based on the disruption of tumor–stroma interaction in secondary lymphoid organs.


Similar content being viewed by others
References
Lenz G, Staudt LM (2010) Aggressive lymphomas. N Engl J Med 362:1417–1429
Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD et al (2010) Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med 362:875–885
Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J et al (2008) Stromal gene signatures in large-B-cell lymphomas. N Engl J Med 359:2313–2323
Mueller SN, Germain RN (2009) Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol 9:618–629
Roozendaal R, Mebius RE (2011) Stromal cell-immune cell interactions. Annu Rev Immunol 29:23–43
Muller G, Lipp M (2003) Concerted action of the chemokine and lymphotoxin system in secondary lymphoid-organ development. Curr Opin Immunol 15:217–224
Muller G, Hopken UE, Lipp M (2003) The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev 195:117–135
Ware CF (2005) Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 23:787–819
Muller G, Hopken UE, Stein H, Lipp M (2002) Systemic immunoregulatory and pathogenic functions of homeostatic chemokine receptors. J Leukoc Biol 72:1–8
Lopez-Giral S, Quintana NE, Cabrerizo M, Alfonso-Perez M, Sala-Valdes M, De Soria VG, Fernandez-Ranada JM, Fernandez-Ruiz E, Munoz C (2004) Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J Leukoc Biol 76:462–471
Mazzucchelli L, Blaser A, Kappeler A, Scharli P, Laissue JA, Baggiolini M, Uguccioni M (1999) BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest 104:R49–54
Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, Kaufmann SH (2007) Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. J Infect Dis 195:46–54
Winter S, Loddenkemper C, Aebischer A, Rabel K, Hoffmann K, Meyer TF, Lipp M, Hopken UE (2010) The chemokine receptor CXCR5 is pivotal for ectopic mucosa-associated lymphoid tissue neogenesis in chronic Helicobacter pylori-induced inflammation. J Mol Med (Berl) 88:1169–1180
Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M (1996) A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell 87:1037–1047
Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23–33
Springer TA (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301–314
Butcher EC, Picker LJ (1996) Lymphocyte homing and homeostasis. Science 272:60–66
Jalkanen S, Reichert RA, Gallatin WM, Bargatze RF, Weissman IL, Butcher EC (1986) Homing receptors and the control of lymphocyte migration. Immunol Rev 91:39–60
Pals ST, de Gorter DJ, Spaargaren M (2007) Lymphoma dissemination: the other face of lymphocyte homing. Blood 110:3102–3111
Drillenburg P, Pals ST (2000) Cell adhesion receptors in lymphoma dissemination. Blood 95:1900–1910
Hopken UE, Foss HD, Meyer D, Hinz M, Leder K, Stein H, Lipp M (2002) Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood 99:1109–1116
Burger JA, Kipps TJ (2002) Chemokine receptors and stromal cells in the homing and homeostasis of chronic lymphocytic leukemia B cells. Leuk Lymphoma 43:461–466
Trentin L, Cabrelle A, Facco M, Carollo D, Miorin M, Tosoni A, Pizzo P, Binotto G, Nicolardi L, Zambello R et al (2004) Homeostatic chemokines drive migration of malignant B cells in patients with non-Hodgkin lymphomas. Blood 104:502–508
Chunsong H, Yuling H, Li W, Jie X, Gang Z, Qiuping Z, Qingping G, Kejian Z, Li Q, Chang AE et al (2006) CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23 + CD5+ B cells. J Immunol 177:6713–6722
Husson H, Freedman AS, Cardoso AA, Schultze J, Munoz O, Strola G, Kutok J, Carideo EG, De Beaumont R, Caligaris-Cappio F et al (2002) CXCL13 (BCA-1) is produced by follicular lymphoma cells: role in the accumulation of malignant B cells. Br J Haematol 119:492–495
Rodig SJ, Jones D, Shahsafaei A, Dorfman DM (2002) CCR6 is a functional chemokine receptor that serves to identify select B-cell non-Hodgkin’s lymphomas. Hum Pathol 33:1227–1233
Rehm A, Anagnostopoulos I, Gerlach K, Broemer M, Scheidereit C, Johrens K, Hubler M, Hetzer R, Stein H, Lipp M, Dorken B, Hopken UE (2009) Identification of a chemokine receptor profile characteristic for mediastinal large B-cell lymphoma. Int J Cancer 125:2367–2374
Kuppers R (2009) The biology of Hodgkin’s lymphoma. Nat Rev Cancer 9:15–27
Yang J, Wang S, Zhao G, Sun B (2011) Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: clinical and experimental study. J Exp Clin Cancer Res 30:51
Carver BS, Pandolfi PP (2006) Mouse modeling in oncologic preclinical and translational research. Clin Cancer Res 12:5305–5311
Tarantul VZ (2004) Transgenic mice as an in vivo model of lymphomagenesis. Int Rev Cytol 236:123–180
Bernardi R, Grisendi S, Pandolfi PP (2002) Modelling haematopoietic malignancies in the mouse and therapeutical implications. Oncogene 21:3445–3458
Seldin DC (1995) New models of lymphoma in transgenic mice. Curr Opin Immunol 7:665–673
Dierks C, Grbic J, Zirlik K, Beigi R, Englund NP, Guo GR, Veelken H, Engelhardt M, Mertelsmann R, Kelleher JF et al (2007) Essential role of stromally induced hedgehog signaling in B-cell malignancies. Nat Med 13:944–951
Refaeli Y, Young RM, Turner BC, Duda J, Field KA, Bishop JM (2008) The B cell antigen receptor and overexpression of MYC can cooperate in the genesis of B cell lymphomas. PLoS Biol 6:e152
Cyster JG (2010) B cell follicles and antigen encounters of the third kind. Nat Immunol 11:989–996
Langdon WY, Harris AW, Cory S, Adams JM (1986) The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell 47:11–18
Reimann M, Loddenkemper C, Rudolph C, Schildhauer I, Teichmann B, Stein H, Schlegelberger B, Dorken B, Schmitt CA (2007) The Myc-evoked DNA damage response accounts for treatment resistance in primary lymphomas in vivo. Blood 110:2996–3004
Reimann M, Schmitt CA, Lee S (2011) Non-cell-autonomous tumor suppression: oncogene-provoked apoptosis promotes tumor cell senescence via stromal crosstalk. J Mol Med (Berl) 89:869–875
Maclean KH, Dorsey FC, Cleveland JL, Kastan MB (2008) Targeting lysosomal degradation induces p53-dependent cell death and prevents cancer in mouse models of lymphomagenesis. J Clin Invest 118:79–88
Ruddell A, Mezquita P, Brandvold KA, Farr A, Iritani BM (2003) B lymphocyte-specific c-Myc expression stimulates early and functional expansion of the vasculature and lymphatics during lymphomagenesis. Am J Pathol 163:2233–2245
Rehm A, Mensen A, Schradi K, Gerlach K, Wittstock S, Winter S, Buchner G, Dorken B, Lipp M, Hopken UE (2011) Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs. Blood 118:1020–1033
Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, Klinakis A, Lukyanov Y, Tseng JC, Sen F et al (2009) CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 459:1000–1004
Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN (2006) Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25:989–1001
Carbone A, Gloghini A, Gruss HJ, Pinto A (1995) CD40 ligand is constitutively expressed in a subset of T cell lymphomas and on the microenvironmental reactive T cells of follicular lymphomas and Hodgkin’s disease. Am J Pathol 147:912–922
Pangault C, Ame-Thomas P, Ruminy P, Rossille D, Caron G, Baia M, De Vos J, Roussel M, Monvoisin C, Lamy T et al (2010) Follicular lymphoma cell niche: identification of a preeminent IL-4-dependent T(FH)-B cell axis. Leukemia 24:2080–2089
Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Briere F, Banchereau J, Caux C (1997) Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med 185:941–951
Craxton A, Magaletti D, Ryan EJ, Clark EA (2003) Macrophage- and dendritic cell-dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood 101:4464–4471
Mueller CG, Boix C, Kwan WH, Daussy C, Fournier E, Fridman WH, Molina TJ (2007) Critical role of monocytes to support normal B cell and diffuse large B cell lymphoma survival and proliferation. J Leukoc Biol 82:567–575
He B, Chadburn A, Jou E, Schattner EJ, Knowles DM, Cerutti A (2004) Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol 172:3268–3279
Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, Gregory CD (2005) Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J Immunol 174:3015–3023
Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66:1–9
Gabrilovich D (2004) Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol 4:941–952
Zou W (2006) Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol 6:295–307
Fricke I, Gabrilovich DI (2006) Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest 35:459–483
Norian LA, Rodriguez PC, O’Mara LA, Zabaleta J, Ochoa AC, Cella M, Allen PM (2009) Tumor-infiltrating regulatory dendritic cells inhibit CD8+ T cell function via L-arginine metabolism. Cancer Res 69:3086–3094
Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK (2007) Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med 204:1037–1047
Janikashvili N, Bonnotte B, Katsanis E, Larmonier N The dendritic cell-regulatory T lymphocyte crosstalk contributes to tumor-induced tolerance. Clin Dev Immunol 2011: 430394
Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB (2010) Immunosuppressive CD14 + HLA-DR(low)/-monocytes in B-cell non-Hodgkin lymphoma. Blood 117:872–881
Maby-El Hajjami H, Ame-Thomas P, Pangault C, Tribut O, DeVos J, Jean R, Bescher N, Monvoisin C, Dulong J, Lamy T et al (2009) Functional alteration of the lymphoma stromal cell niche by the cytokine context: role of indoleamine-2,3 dioxygenase. Cancer Res 69:3228–3237
Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, Jean R, Monnier D, Caulet-Maugendre S, Guillaudeux T, Lamy T, Fest T, Tarte K (2007) Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood 109:693–702
Krampera M (2011) Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 25:1408–1414
Chiorazzi N, Rai KR, Ferrarini M (2005) Chronic lymphocytic leukemia. N Engl J Med 352:804–815
Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F (2009) The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 114:3367–3375
Lagneaux L, Delforge A, Bron D, De Bruyn C, Stryckmans P (1998) Chronic lymphocytic leukemic B cells but not normal B cells are rescued from apoptosis by contact with normal bone marrow stromal cells. Blood 91:2387–2396
Vincent AM, Cawley JC, Burthem J (1996) Integrin function in chronic lymphocytic leukemia. Blood 87:4780–4788
Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG (2000) Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 97:12694–12699
Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, Bremer J, Iezzi G, Graf R, Clavien PA et al (2009) A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell 16:295–308
Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M (2010) B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature 464:302–305
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Browning JL (2008) Inhibition of the lymphotoxin pathway as a therapy for autoimmune disease. Immunol Rev 223:202–220
Wolf MJ, Seleznik GM, Zeller N, Heikenwalder M (2010) The unexpected role of lymphotoxin beta receptor signaling in carcinogenesis: from lymphoid tissue formation to liver and prostate cancer development. Oncogene 29:5006–5018
Zenz T, Mertens D, Kuppers R, Dohner H, Stilgenbauer S (2010) From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer 10:37–50
Burger JA (2011) Nurture versus nature: the microenvironment in chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Program 2011:96–103
Burger JA (2010) Chemokines and chemokine receptors in chronic lymphocytic leukemia (CLL): from understanding the basics towards therapeutic targeting. Semin Cancer Biol 20:424–430
Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, Schaefer-Cutillo J, De Vos S, Sinha R, Leonard JP et al (2010) Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 115:2578–2585
Honigberg LA, Smith AM, Sirisawad M, Verner E, Loury D, Chang B, Li S, Pan Z, Thamm DH, Miller RA et al (2010) The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA 107:13075–13080
Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M et al (2011) CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117:591–594
Quiroga MP, Balakrishnan K, Kurtova AV, Sivina M, Keating MJ, Wierda WG, Gandhi V, Burger JA (2009) B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood 114:1029–1037
Hu Y, Gale M, Shields J, Garron C, Swistak M, Nguyen TH, Jacques G, Fogle R, Siders W, Kaplan J (2012) Enhancement of the anti-tumor activity of therapeutic monoclonal antibodies by CXCR4 antagonists. Leuk Lymphoma 53:130–138
O’Callaghan K, Lee L, Nguyen N, Hsieh MY, Kaneider NC, Klein AK, Sprague K, Van Etten RA, Kuliopulos A, Covic L (2012) Targeting CXCR4 with cell-penetrating pepducins in lymphoma and lymphocytic leukemia. Blood 119:1717–1725
Forster R, Davalos-Misslitz AC, Rot A (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8:362–371
Winter S, Rehm A, Wichner K, Scheel T, Batra A, Siegmund B, Berek C, Lipp M, Hopken UE (2011) Manifestation of spontaneous and early autoimmune gastritis in CCR7-deficient mice. Am J Pathol 179:754–765
Acknowledgments
We are grateful to Martin Lipp and Bernd Dörken for helpful discussions and we thank Kristina Schradi for critical reading of the manuscript. This work was supported by the German Cancer Foundation and the Berliner Krebshilfe.
Conflict of interest statement
The authors declare that they have no conflict of interests or financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Höpken, U.E., Rehm, A. Homeostatic chemokines guide lymphoma cells to tumor growth-promoting niches within secondary lymphoid organs. J Mol Med 90, 1237–1245 (2012). https://doi.org/10.1007/s00109-012-0906-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0906-z