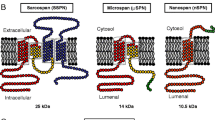
Abstract
Selenoprotein N (SelN) deficiency causes several inherited neuromuscular disorders collectively termed SEPN1-related myopathies, characterized by early onset, generalized muscle atrophy, and muscle weakness affecting especially axial muscles and leading to spine rigidity, severe scoliosis, and respiratory insufficiency. SelN is ubiquitously expressed and is located in the membrane of the endoplasmic reticulum; however, its function remains elusive. The predominant expression of SelN in human fetal tissues and the embryonic muscle phenotype reported in mutant zebrafish suggest that it is involved in myogenesis. In mice, SelN is also mostly expressed during embryogenesis and especially in the myotome, but no defect was detected in muscle development and growth in the Sepn1 knock-out mouse model. By contrast, we recently demonstrated that SelN is essential for muscle regeneration and satellite cell maintenance in mice and humans, hence opening new avenues regarding the pathomechanism(s) leading to SEPN1-related myopathies. At the cellular level, recent data suggested that SelN participates in oxidative and calcium homeostasis, with a potential role in the regulation of the ryanodine receptor activity. Despite the recent and exciting progress regarding the physiological function(s) of SelN in muscle tissue, the pathogenesis leading to SEPN1-related myopathies remains largely unknown, with several unsolved questions, and no treatment available. In this review, we introduce SelN, its properties and expression pattern in zebrafish, mice, and humans, and we discuss its potential roles in muscle tissue and the ensuing clues for the development of therapeutic options.





Similar content being viewed by others
References
Pinsent J (1954) The need for selenite and molybdate in the formation of formic dehydrogenase by members of the Coli-aerogenes group of bacteria. Biochem J 57:10–16
Jacob C, Giles GI, Giles NM, Sies H (2003) Sulfur and selenium: the role of oxidation state in protein structure and function. Angew Chem Int Ed Engl 42:4742–4758
Patching SG, Gardiner PH (1999) Recent developments in selenium metabolism and chemical speciation: a review. J Trace Elem Med Biol 13:193–214
Andreesen JR, Ljungdahl LG (1973) Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol 116:867–873
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179:588–590
Yang GQ, Wang SZ, Zhou RH, Sun SZ (1983) Endemic selenium intoxication of humans in China. Am J Clin Nutr 37:872–881
Fan AM, Kizer KW (1990) Selenium. Nutritional, toxicologic, and clinical aspects. West J Med 153:160–167
Bellinger FP, Raman AV, Reeves MA, Berry MJ (2009) Regulation and function of selenoproteins in human disease. Biochem J 422:11–22
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806
Lobanov AV, Hatfield DL, Gladyshev VN (2009) Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta 1790:1424–1428
Rayman MP, Infante HG, Sargent M (2008) Food-chain selenium and human health: spotlight on speciation. Br J Nutr 100:238–253
Rederstorff M, Krol A, Lescure A (2006) Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci 63:52–59
Lescure A, Rederstorff M, Krol A, Guicheney P, Allamand V (2009) Selenoprotein function and muscle disease. Biochim Biophys Acta 1790:1569–1574
Zhuo P, Diamond AM (2009) Molecular mechanisms by which selenoproteins affect cancer risk and progression. Biochim Biophys Acta 1790:1546–1554
Rayman MP (2000) The importance of selenium to human health. Lancet 356:233–241
Brigelius-Flohe R, Kipp A (2009) Glutathione peroxidases in different stages of carcinogenesis. Biochim Biophys Acta 1790:1555–1568
Sturchler C, Westhof E, Carbon P, Krol A (1993) Unique secondary and tertiary structural features of the eucaryotic selenocysteine tRNA(Sec). Nucleic Acids Res 21:1073–1079
Hubert N, Walczak R, Carbon P, Krol A (1996) A protein binds the selenocysteine insertion element in the 3′-UTR of mammalian selenoprotein mRNAs. Nucleic Acids Res 24:464–469
Lesoon A, Mehta A, Singh R, Chisolm GM, Driscoll DM (1997) An RNA-binding protein recognizes a mammalian selenocysteine insertion sequence element required for cotranslational incorporation of selenocysteine. Mol Cell Biol 17:1977–1985
Allmang C, Wurth L, Krol A (2009) The selenium to selenoprotein pathway in eukaryotes: more molecular partners than anticipated. Biochim Biophys Acta 1790:1415–1423
Howard MT, Aggarwal G, Anderson CB, Khatri S, Flanigan KM, Atkins JF (2005) Recoding elements located adjacent to a subset of eukaryal selenocysteine-specifying UGA codons. EMBO J 24:1596–1607
Maiti B, Arbogast S, Allamand V, Moyle MW, Anderson CB, Richard P, Guicheney P, Ferreiro A, Flanigan KM, Howard MT (2009) A mutation in the SEPN1 selenocysteine redefinition element (SRE) reduces selenocysteine incorporation and leads to SEPN1-related myopathy. Hum Mutat 30:411–416
Fagegaltier D, Hubert N, Yamada K, Mizutani T, Carbon P, Krol A (2000) Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J 19:4796–4805
Copeland PR, Driscoll DM (1999) Purification, redox sensitivity, and RNA binding properties of SECIS-binding protein 2, a protein involved in selenoprotein biosynthesis. J Biol Chem 274:25447–25454
Halic M, Becker T, Frank J, Spahn CM, Beckmann R (2005) Localization and dynamic behavior of ribosomal protein L30e. Nat Struct Mol Biol 12:467–468
Kinzy SA, Caban K, Copeland PR (2005) Characterization of the SECIS binding protein 2 complex required for the co-translational insertion of selenocysteine in mammals. Nucleic Acids Res 33:5172–5180
Arner ES (2010) Selenoproteins—what unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res 316:1296–1303
Lee SR, Bar-Noy S, Kwon J, Levine RL, Stadtman TC, Rhee SG (2000) Mammalian thioredoxin reductase: oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc Natl Acad Sci USA 97:2521–2526
Zhong L, Arner ES, Holmgren A (2000) Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine–selenocysteine sequence. Proc Natl Acad Sci USA 97:5854–5859
Bar-Noy S, Gorlatov SN, Stadtman TC (2001) Overexpression of wild type and SeCys/Cys mutant of human thioredoxin reductase in E. coli: the role of selenocysteine in the catalytic activity. Free Radic Biol Med 30:51–61
Arner ES (2009) Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta 1790:495–526
Steinbrenner H, Sies H (2009) Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta 1790:1478–1485
Lescure A, Gautheret D, Carbon P, Krol A (1999) Novel selenoproteins identified in silico and in vivo by using a conserved RNA structural motif. J Biol Chem 274:38147–38154
Lescure A, Castets P, Grunwald D, Allamand V, Howard M (2012) Selenoprotein N: its role in disease. In: Hatfield DL, Berry MJ, Gladyshev VN (eds) Selenium: its molecular biology and role in human health. Springer, Berlin, pp 283–294
Moghadaszadeh B, Petit N, Jaillard C, Brockington M, Roy SQ, Merlini L, Romero N, Estournet B, Desguerre I, Chaigne D et al (2001) Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet 29:17–18
Schmid CW, Jelinek WR (1982) The Alu family of dispersed repetitive sequences. Science 216:1065–1070
Makalowski W, Mitchell GA, Labuda D (1994) Alu sequences in the coding regions of mRNA: a source of protein variability. Trends Genet 10:188–193
Petit N, Lescure A, Rederstorff M, Krol A, Moghadaszadeh B, Wewer UM, Guicheney P (2003) Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum Mol Genet 12:1045–1053
Lin L, Shen S, Tye A, Cai JJ, Jiang P, Davidson BL, Xing Y (2008) Diverse splicing patterns of exonized Alu elements in human tissues. PLoS Genet 4:e1000225
Mayer BJ (2001) SH3 domains: complexity in moderation. J Cell Sci 114:1253–1263
Li SS (2005) Specificity and versatility of SH3 and other proline-recognition domains: structural basis and implications for cellular signal transduction. Biochem J 390:641–653
Shchedrina VA, Zhang Y, Labunskyy VM, Hatfield DL, Gladyshev VN (2010) Structure–function relations, physiological roles, and evolution of mammalian ER-resident selenoproteins. Antioxid Redox Signal 12:839–849
Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, Counsell S, Guicheney P, Muntoni F, Merlini L (2002) Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1). Neuromuscul Disord 12:631–638
Arkader A, Hosalkar H, Dormans JP (2005) Scoliosis correction in an adolescent with a rigid spine syndrome: case report. Spine (Phila Pa 1976) 30:E623–E628
Mercuri E, Clements E, Offiah A, Pichiecchio A, Vasco G, Bianco F, Berardinelli A, Manzur A, Pane M, Messina S et al (2010) Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol 67:201–208
Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, Manson JI, Kornberg AJ, Shield LK, North KN (2006) SEPN1: associated with congenital fiber-type disproportion and insulin resistance. Ann Neurol 59:546–552
Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, Gladyshev VN (2011) Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal 14:2327–2336
Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM et al (2009) Reactive oxygen species enhance insulin sensitivity. Cell Metab 10:260–272
Lei XG, Vatamaniuk MZ (2011) Two tales of antioxidant enzymes on β cells and diabetes. Antioxid Redox Signal 14:489–503. doi:10.1089/ars.2010.3416
Scoto M, Cirak S, Mein R, Feng L, Manzur AY, Robb S, Childs AM, Quinlivan RM, Roper H, Jones DH et al (2011) SEPN1-related myopathies: clinical course in a large cohort of patients. Neurology 76:2073–2078
Cagliani R, Fruguglietti ME, Berardinelli A, D'Angelo MG, Prelle A, Riva S, Napoli L, Gorni K, Orcesi S, Lamperti C et al (2011) New molecular findings in congenital myopathies due to selenoprotein N gene mutations. J Neurol Sci 300:107–113
Moghadaszadeh B, Desguerre I, Topaloglu H, Muntoni F, Pavek S, Sewry C, Mayer M, Fardeau M, Tome FM, Guicheney P (1998) Identification of a new locus for a peculiar form of congenital muscular dystrophy with early rigidity of the spine, on chromosome 1p35–36. Am J Hum Genet 62:1439–1445
Flanigan KM, Kerr L, Bromberg MB, Leonard C, Tsuruda J, Zhang P, Gonzalez-Gomez I, Cohn R, Campbell KP, Leppert M (2000) Congenital muscular dystrophy with rigid spine syndrome: a clinical, pathological, radiological, and genetic study. Ann Neurol 47:152–161
Tajsharghi H, Darin N, Tulinius M, Oldfors A (2005) Early onset myopathy with a novel mutation in the selenoprotein N gene (SEPN1). Neuromuscul Disord 15:299–302
Okamoto Y, Takashima H, Higuchi I, Matsuyama W, Suehara M, Nishihira Y, Hashiguchi A, Hirano R, Ng AR, Nakagawa M et al (2006) Molecular mechanism of rigid spine with muscular dystrophy type 1 caused by novel mutations of selenoprotein N gene. Neurogenetics 7:175–183
Moghadaszadeh B, Topaloglu H, Merlini L, Muntoni F, Estournet B, Sewry C, Naom I, Barois A, Fardeau M, Tome FM et al (1999) Genetic heterogeneity of congenital muscular dystrophy with rigid spine syndrome. Neuromuscul Disord 9:376–382
Ferreiro A, Ceuterick-de Groote C, Marks JJ, Goemans N, Schreiber G, Hanefeld F, Fardeau M, Martin JJ, Goebel HH, Richard P et al (2004) Desmin-related myopathy with Mallory body-like inclusions is caused by mutations of the selenoprotein N gene. Ann Neurol 55:676–686
Ferreiro A, Quijano-Roy S, Pichereau C, Moghadaszadeh B, Goemans N, Bönnemann C, Jungbluth H, Straub V, Villanova M, Leroy JP et al (2002) Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet 71:739–749
Sponholz S, von der Hagen M, Hahn G, Seifert J, Richard P, Stoltenburg-Didinger G, Ferreiro A, Kaindl AM (2006) Selenoprotein N muscular dystrophy: differential diagnosis for early-onset limited mobility of the spine. J Child Neurol 21:316–320
Herasse M, Parain K, Marty I, Monnier N, Kaindl AM, Leroy JP, Richard P, Lunardi J, Romero NB, Ferreiro A (2007) Abnormal distribution of calcium-handling proteins: a novel distinctive marker in core myopathies. J Neuropathol Exp Neurol 66:57–65
Schara U, Kress W, Bönnemann CG, Breitbach-Faller N, Korenke CG, Schreiber G, Stoetter M, Ferreiro A, von der Hagen M (2008) The phenotype and long-term follow-up in 11 patients with juvenile selenoprotein N1-related myopathy. Eur J Paediatr Neurol 12:224–230
Boyden SE, Salih MA, Duncan AR, White AJ, Estrella EA, Burgess SL, Seidahmed MZ, Al-Jarallah AS, Alkhalidi HM, Al-Maneea WM et al (2010) Efficient identification of novel mutations in patients with limb girdle muscular dystrophy. Neurogenetics 11:449–455
Rederstorff M, Allamand V, Guicheney P, Gartioux C, Richard P, Chaigne D, Krol A, Lescure A (2008) Ex vivo correction of selenoprotein N deficiency in rigid spine muscular dystrophy caused by a mutation in the selenocysteine codon. Nucleic Acids Res 36:237–244
Allamand V, Richard P, Lescure A, Ledeuil C, Desjardin D, Petit N, Gartioux C, Ferreiro A, Krol A, Pellegrini N et al (2006) A single homozygous point mutation in a 3′untranslated region motif of selenoprotein N mRNA causes SEPN1-related myopathy. EMBO Rep 7:450–454
Schoenmakers E, Agostini M, Mitchell C, Schoenmakers N, Papp L, Rajanayagam O, Padidela R, Ceron-Gutierrez L, Doffinger R, Prevosto C et al (2010) Mutations in the selenocysteine insertion sequence-binding protein 2 gene lead to a multisystem selenoprotein deficiency disorder in humans. J Clin Invest 120:4220–4235
Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S (2005) Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252
Park SM, Chatterjee VK (2005) Genetics of congenital hypothyroidism. J Med Genet 42:379–389
Di Cosmo C, McLellan N, Liao XH, Khanna KK, Weiss RE, Papp L, Refetoff S (2009) Clinical and molecular characterization of a novel selenocysteine insertion sequence-binding protein 2 (SBP2) gene mutation (R128X). J Clin Endocrinol Metab 94:4003–4009
Thisse C, Degrave A, Kryukov GV, Gladyshev VN, Obrecht-Pflumio S, Krol A, Thisse B, Lescure A (2003) Spatial and temporal expression patterns of selenoprotein genes during embryogenesis in zebrafish. Gene Expr Patterns 3:525–532
Jurynec MJ, Xia R, Mackrill JJ, Gunther D, Crawford T, Flanigan KM, Abramson JJ, Howard MT, Grunwald DJ (2008) Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle. Proc Natl Acad Sci USA 105:12485–12490
Deniziak M, Thisse C, Rederstorff M, Hindelang C, Thisse B, Lescure A (2007) Loss of selenoprotein N function causes disruption of muscle architecture in the zebrafish embryo. Exp Cell Res 313:156–167
Castets P, Maugenre S, Gartioux C, Rederstorff M, Krol A, Lescure A, Tajbakhsh S, Allamand V, Guicheney P (2009) Selenoprotein N is dynamically expressed during mouse development and detected early in muscle precursors. BMC Dev Biol 9:46
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ (2006) The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38:228–233
Rederstorff M, Castets P, Arbogast S, Lainé J, Vassilopoulos S, Beuvin M, Dubourg O, Vignaud A, Ferry A, Krol A et al (2011) Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy. PLoS One 6(8):e23094
Tajbakhsh S (2009) Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med 266:372–389
Castets P, Bertrand AT, Beuvin M, Ferry A, Le Grand F, Castets M, Chazot G, Rederstorff M, Krol A, Lescure A et al (2011) Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum Mol Genet 20:694–704
Kirkland JL, Tchkonia T, Pirtskhalava T, Han J, Karagiannides I (2002) Adipogenesis and aging: does aging make fat go MAD? Exp Gerontol 37:757–767
Taylor-Jones JM, McGehee RE, Rando TA, Lecka-Czernik B, Lipschitz DA, Peterson CA (2002) Activation of an adipogenic program in adult myoblasts with age. Mech Ageing Dev 123:649–661
Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA (1995) Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem 270:28183–28187
Oguro H, Iwama A (2007) Life and death in hematopoietic stem cells. Curr Opin Immunol 19:503–509
Kim BS, Jung JS, Jang JH, Kang KS, Kang SK (2011) Nuclear Argonaute 2 regulates adipose tissue-derived stem cell survival through direct control of miR10b and selenoprotein N1 expression. Aging Cell 10:277–291
Go YM, Jones DP (2008) Redox compartmentalization in eukaryotic cells. Biochim Biophys Acta 1780:1273–1290
Stoytcheva ZR, Berry MJ (2009) Transcriptional regulation of mammalian selenoprotein expression. Biochim Biophys Acta 1790:1429–1440
Arbogast S, Ferreiro A (2010) Selenoproteins and protection against oxidative stress: selenoprotein N as a novel player at the crossroads of redox signaling and calcium homeostasis. Antioxid Redox Signal 12:893–904
Arbogast S, Beuvin M, Fraysse B, Zhou H, Muntoni F, Ferreiro A (2009) Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann Neurol 65:677–686
Moghadaszadeh B, Aracena-Parks P, Ronan M, Gasmi H, Agrawal P, Hamilton S, Beggs A (2007) SEPN1-related myopathy: a defect in redox regulation. Neuromuscul Disord 17:899–900
Gao Y, Feng HC, Walder K, Bolton K, Sunderland T, Bishara N, Quick M, Kantham L, Collier GR (2004) Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress—SelS is a novel glucose-regulated protein. FEBS Lett 563:185–190
Shchedrina VA, Everley RA, Zhang Y, Gygi SP, Hatfield DL, Gladyshev VN (2011) Selenoprotein K binds multiprotein complexes and is involved in the regulation of endoplasmic reticulum homeostasis. J Biol Chem 286:42937–42948
Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN (2001) Association between the 15-kDa selenoprotein and UDP-glucose:glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem 276:15330–15336
Porter Moore C, Zhang JZ, Hamilton SL (1999) A role for cysteine 3635 of RYR1 in redox modulation and calmodulin binding. J Biol Chem 274:36831–36834
Oba T, Kurono C, Nakajima R, Takaishi T, Ishida K, Fuller GA, Klomkleaw W, Yamaguchi M (2002) H2O2 activates ryanodine receptor but has little effect on recovery of releasable Ca2+ content after fatigue. J Appl Physiol 93:1999–2008
Hatfield DL, Yoo MH, Carlson BA, Gladyshev VN (2009) Selenoproteins that function in cancer prevention and promotion. Biochim Biophys Acta 1790:1541–1545
Grumolato L, Ghzili H, Montero-Hadjadje M, Gasman S, Lesage J, Tanguy Y, Galas L, Ait-Ali D, Leprince J, Guerineau NC et al (2008) Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 22:1756–1768
Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120:85–98
Li Y, Camacho P (2004) Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol 164:35–46
Pisaniello A, Serra C, Rossi D, Vivarelli E, Sorrentino V, Molinaro M, Bouche M (2003) The block of ryanodine receptors selectively inhibits fetal myoblast differentiation. J Cell Sci 116:1589–1597
Daiho T, Kanazawa T (1994) Reduction of disulfide bonds in sarcoplasmic reticulum Ca(2+)-ATPase by dithiothreitol causes inhibition of phosphoenzyme isomerization in catalytic cycle. This reduction requires binding of both purine nucleotide and Ca2+ to enzyme. J Biol Chem 269:11060–11064
Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C (1999) Protein modification during biological aging: selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J 340(Pt 3):657–669
Viner RI, Krainev AG, Williams TD, Schoneich C, Bigelow DJ (1997) Identification of oxidation-sensitive peptides within the cytoplasmic domain of the sarcoplasmic reticulum Ca2+-ATPase. Biochemistry 36:7706–7716
Roderick HL, Bootman MD (2005) Redoxing calcium from the ER. Cell 120:4–5
Berchtold MW, Brinkmeier H, Muntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80:1215–1265
Moylan JS, Reid MB (2007) Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 35:411–429
Acknowledgments
We acknowledge funding from the Institut National de la Santé et de la Recherche Médicale (Inserm), Association Française contre les Myopathies (AFM), UPMC Université Paris 06, Centre National de la Recherche Scientifique (CNRS), Assistance Publique-Hôpitaux de Paris (AP-HP). We thank Dr. Rachel Peat for English proofing. We apologize to all colleagues who might have not been cited in this review due to space limitation.
Competing interests
None is declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castets, P., Lescure, A., Guicheney, P. et al. Selenoprotein N in skeletal muscle: from diseases to function. J Mol Med 90, 1095–1107 (2012). https://doi.org/10.1007/s00109-012-0896-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0896-x