Abstract
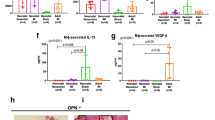
Following a severe ischemic injury or myocardial infarction, the extracellular matrix (ECM) of the heart is involved in pathophysiological conditions such as dilatation and cardiac dysfunction. Osteopontin (OPN) has been shown to interact with fibronectin suggesting its possible role in matrix organization, stability and wound healing. There is increased expression of OPN in several tissues in response to injury. Therefore, we tested the hypothesis that acute ischemia (2 h), followed by reperfusion (4 h) may induce early OPN and fibronectin in an isolated hemoperfused working porcine heart model. Twenty hearts were prepared and connected to a perfusion system. After 1 h of perfusion, these hearts were randomized to two groups: ten infarcted (MI, ramus circumflexus) and ten non-infarcted hearts (C). In addition, cardiac fibroblasts derived from infarcted, remote and control myocardium were investigated. In both groups, the heart rate, electrolytes, pH, blood gases, and lactate remained similar. The LVEDP and perfusion pressure of MI hearts increased significantly (P<0.05). The total fibronectin and OPN volume contents were clearly elevated in the infarct area. The matrix metalloproteinases (MMP-1 and MMP-8), fibronectin, OPN, TGF-β1 proteins and the mRNAs for fibronectin, TGF-β1, and OPN were significantly elevated in the infarct area as compared to the remote area and the non-infarcted hearts. Simultaneously, circulating carboxyterminal propeptide of type I procollagen (PICP) was released in the perfusion medium (threefold versus C). Fibroblast-like cells originating from the infarct area exhibited an enhanced OPN and fibronectin gene and protein expression compared to fibroblasts derived from control myocardium. Our data demonstrate the early appearance of the MMPs (increased collagen degrading enzymes) and PICP (a collagen synthesis marker) following ischemia and reperfusion. Moreover, OPN, fibronectin and TGF-β1 protein and gene expression are elevated after ischemia and reperfusion in the ex vivo working hemoperfused porcine heart model.






Similar content being viewed by others
References
Ho KK, Pinsky JL, Kannel WB, Levy D (1993) The epidemiology of heart failure: the Framingham study. J Am Coll Cardiol 22:6A–13A
Murray CJ, Lopez AD (1997) Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet 349:1498–1504
Grimm D, Cameron D, Griese DP, Riegger GAJ, Kromer EP (1998) Differential effects of growth hormone on cardiomyocyte and extracellular matrix protein remodeling following experimental myocardial infarction. Cardiovasc Res 40:297–306
Takino T, Nakamura M, Hiramori K (1999) Circulating levels of carboxyterminal propeptide of type I procollagen and left ventricular remodeling after myocardial infarction. Cardiology 91:81–86
John-Sutton MG St, Sharpe N (2000) Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation 101:2981–2988
Wiggers H, Klebe T, Heickendorf L, Host NB, Danielsen CC, Baandrup U, Andersen HR (1997) Ischemia and reperfusion of the porcine myocardium: effect on collagen. J Mol Cell Cardiol 29:289–299
Frangogiannis NG, Youker KA, Rossen RD, Gwechenberger M, Lindsey MH, Mendoza LH, Michael LH, Ballantyne CM, Smith CW, Entman ML (1998) Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol 30:2567–2576
Lee RT, Lammerding J (2002) Signaling pathways that influence extracellular remodeling. J Card Fail 8:S339–S343
Grimm D, Huber M, Jabusch HC, Shakibaei M, Fredersdorf S, Paul M, Riegger GAJ, Kromer EP (2001) Extracellular matrix proteins in cardiac fibroblasts derived from rat hearts with chronic pressure overload: effects of beta-receptor blockade. J Mol Cell Cardiol 33:487–501
Nogueira AC, Ast I, Patone G, Perschel FH, Grimm D, Paul M (2003) Functional effects of acute coronary occlusion and catecholinergic stimuli on the isolated normothermic hemoperfused porcine heart. Clin Exp Hypertens 25:235–255
Whittacker P (1995) Unravelling the mysteries of collagen and cicatrix after myocardial infarction. Cardiovasc Res 29:758–762
Takahashi S, Barry AC, Factor S (1990) Collagen degradation in ischemic rat hearts. Biochem J 265:233–241
Factor SM, Robinson TF, Dominitz R, Cho S (1986) Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture. Am J Cardiovasc Pathol 1:91–97
Casscells W, Kimura H, Sanchez JA, Yu ZX, Ferrans VJ (1990) Immunhistochemical study of fibronectin in experimental myocardial infarction. Am J Pathol 137:801–810
Serebruany VL, Solomon SR, Herzog WR, Gurbel PA (1998) Plasma fibronectin during myocardial ischemia-reperfusion: effects of magnesium, diltiazem, and a novel MAC-1 inhibitor. Am J Hematol 57:309–314
Schnee JM, Hsueh WA (2000) Angiotensin II, adhesion, and cardiac fibrosis. Cardiovasc Res 46:264–268
Rothermund L, Kreutz R, Kossmehl P, Fredersdorf S, Shakibaei M, Schulze-Tanzil G, Paul M, Grimm D (2002) Early onset of chondroitin sulfate and OPN expression in angiotensin II-dependent left ventricular hypertrophy. Am J Hypertens 15:644–652
Hupf H, Grimm D, Riegger GAJ, Schunkert H (1999) Evidence for a vasopressin system in the rat heart. Circ Res 84:365–370
Kimose HH, Helligso P, Randsbaek F, Kim Y, Botker HE, Hansen SB, Thomassen AR, Nielsen TT (1996) Improved recovery after cold crystalloid cardioplegia using low-dose glutamate enrichment during reperfusion after aortic unclamping: a study in isolated blood-perfused pig hearts. Thorac Cardiovasc Surg 44:118–125
Horstick G, Heimann A, Gotze O, Hafner G, Berg O, Boehmer P, Becker P, Darius H, Rupprecht HJ, Loos M, Bhakdi S, Meyer J, Kempski O (1997) Intracoronary application of C1 esterase inhibitor improves cardiac function and reduces myocardial necrosis in an experimental model of ischemia and reperfusion. Circulation 95:701–708
Wai PY, Kuo PC (2004) The role of osteopontin in tumor metastasis. J Surg Res 121:228–241
Komatsubara I, Murakami T, Kusachi S, Nakamura K, Hirohata S, Hayashi J, Takemoto S, Suezawa C, Ninomiya Y, Shiratori Y (2003) Spatially and temporally different expression of osteonectin and OPN in the infarct zone of experimentally induced myocardial infarction in rats. Cardiovasc Pathol 12:186–194
Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS (1999) Myocardial OPN expression coincides with the development of heart failure. Hypertension 33:663–670
Mukherjee BB, Nemir M, Beninati S, Cordella-Miele E, Singh K, Chackalaparampil I, Shanmugam V, DeVouge MW, Mukherjee AB (1995) Interaction of osteopontin with fibronectin and other extracellular matrix molecules. Ann N Y Acad Sci 760:201–212
Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K (2001) Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking OPN. Circ Res 88:1080–1087
Cleutjens JP, Creemers EE (2002) Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J Card Fail 8:S344–S348
Fraccarollo D, Galuppo P, Bauersachs J, Ertl G (2002) Collagen accumulation after myocardial infarction: effects of ETA receptor blockade and implications for early remodeling. Cardiovasc Res 54:559–567
Pfeffer MA (1995) Left ventricular remodeling after acute myocardial infarction. Annu Rev Med 46:455–466
Chen H, Li D, Saldeen T, Mehta JL (2003) TGF-beta 1 attenuates myocardial ischemia-reperfusion injury via inhibition of upregulation of MMP-1. Am J Physiol Heart Circ Physiol 284:H1612–H1617
Danielsen CC, Wiggers H, Andersen HR (1998) Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J Mol Cell Cardiol 30:1431–1442
Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH III, Edmunds LH Jr, Gorman RC, Spinale FG (2003) Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation 107:2857–2863
Inokubo Y, Hanada H, Ishizaka H, Fukushi T, Kamada T, Okumura K (2001) Plasma levels of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 are increased in the coronary circulation in patients with acute coronary syndrome. Am Heart J 141:211–217
Lefer AM, Tsao P, Aoki N, Palladino MA Jr (1990) Mediation of cardioprotection by transforming growth factor-beta. Science 249:61–64
Baxter GF, Mocanu MM, Brar BK, Latchman DS, Yellon DM (2001) Cardioprotective effects of transforming growth factor-beta1 during early reoxygenation or reperfusion by p42/p44 MAPK. J Cardiovasc Pharmacol 38:930–939
Wilson EM, Spinale FG (2001) Myocardial remodelling and matrix metalloproteinases in heart failure: turmoil within the interstitium. Ann Med 33:623–634
Kostin S, Rieger M, Dammer S, Hein S, Richter M, Klovekorn W, Bauer EP, Schaper J (2003) Gap junction remodeling and altered connexin 43 expression in the failing human heart. Mol Cell Biochem 242:135–144
Delcayre C, Swynghedauw B (2002) Molecular mechanisms of myocardial remodeling. The role of aldosterone. J Mol Cell Cardiol 34:1577–1584
Spinale FG, Gunasinghe H, Sprunger PD, Baskin JM, Bradham WC (2002) Extracellular degradative pathways in myocardial remodeling and progression to heart failure. J Card Fail 8:S332–S338
Cannon RO III, Butany JW, McManus BM, Speir E, Kravitz AB, Bolli R, Ferrans VJ (1983) Early degradation of collagen after acute myocardial infarction in the rat. Am J Cardiol 52:390–395
Charney RH, Takahashi S, Zhao M, Sonnenblick EH, Eng C (1992) Collagen loss in the stunned myocardium. Circulation 85:1483–1490
Whittaker P, Boughner DR, Kloner RA (1991) Role of collagen in acute myocardial infarct expansion. Circulation 84:2123–2134
Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P (1992) Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest 89:1060–1068
Grinnell F (1984) Fibronectin and wound healing. J Cell Biochem 26:107–116
Contard F, Koteliansky V, Marotte F, Dubus I, Rappaprot L, Samuel JL (1991) Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest 64:65–67
Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT (1995) Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol 27:1281–1292
Author information
Authors and Affiliations
Corresponding author
Additional information
This research was supported by the Deutsche Forschungsgemeinschaft (DFG) grants GR 1039/7-1 and GR 1039/7-2 to D.G.
Rights and permissions
About this article
Cite this article
Kossmehl, P., Schönberger, J., Shakibaei, M. et al. Increase of fibronectin and osteopontin in porcine hearts following ischemia and reperfusion. J Mol Med 83, 626–637 (2005). https://doi.org/10.1007/s00109-005-0642-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-005-0642-8