Abstract
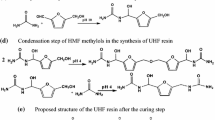
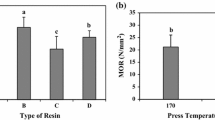
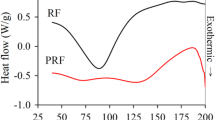
Amino resin precursors prepared by the addition of a new, colourless, non-volatile and non-toxic aldehyde, dimethoxyethanal (DME), to urea gave resins for boards that while able to harden were underperforming due to the lower reactivity of DME in relation to formaldehyde. Urea reacts with one and even two molecules of DME to form UDME and U(DME)2 (called DU) but the subsequent cross-linking reaction to form bridges between two ureas, although existing as observed by CP MAS 13C NMR, was too slow at temperatures lower than 140 °C to be of significance for wood panel adhesives. However, addition of 20% isocyanate (pMDI) contributed to cross-linking of DU by its reaction with pMDI to also form urethane bridges, their existence being confirmed by CP-MAS 13C NMR. The adhesive resins so formed had excellent performance, were colourless, and produced boards that satisfied well the requirements of the relevant norms for interior panels (EN 120 and EN 312). The results were good enough to decrease the proportion of pMDI to 14% at pressing times starting to be of significance for industrial panel products. Formaldehyde emission, by perforator method was down exclusively to the formaldehyde produced by heating the wood chips. The panel emission was sufficiently low to even satisfy the relevant F**** JIS A 5908 Japanese standard (JIS A 5908, 1994 ). These adhesives are colourless, as UF resins.
Zusammenfassung
Durch Zugabe von Dimethoxyethanal (DME), einem neuen, farblosen, nicht flüchtigen und ungiftigen Aldehyd, zu Harnstoff, wurden Vorstufen von Aminoharz hergestellt. Dies ergab Harze, die zwar härteten, jedoch aufgrund der im Vergleich zu Formaldehyd geringeren Reaktivität von DME die Erwartungen nicht ganz erfüllten. Harnstoff reagiert mit einem oder auch zwei DME-Molekülen und bildet UDME und U(DME)2 (als DU bezeichnet). Die nachfolgende Vernetzungsreaktion zur Bildung von Brücken zwischen zwei Harnstoffen war zwar, wie anhand CP-MAS 13CNMR gezeigt wurde, vorhanden, bei Temperaturen unter 140 °C war sie jedoch zu langsam, um die Anforderungen an Klebstoffe für Holzwerkstoffe zu erfüllen. Mittels CP-MAS 13CNMR konnte nachgewiesen werden, dass durch Zugabe von 20% Isocyanat (pMDI) die Vernetzung von DU durch Reaktion mit pMDI durch zusätzliche Bildung von Urethan-Brücken verbessert wurde. Die so hergestellten Harze zeigten ausgezeichnete Eigenschaften, sie waren farblos und ergaben Platten, die die Anforderungen der entsprechenden Normen für Platten im Innenbereich (EN 120 und 312) erfüllten. Die Ergebnisse waren so gut, dass der pMDI-Anteil auf 14% verringert werden konnte bei Presszeiten, die für eine industrielle Produktion noch akzeptabel sind. Die mittels Perforatormethode ermittelte Formaldehydemission hing ausschließlich von dem beim Erhitzen der Holzspäne entstandenen Formaldehyd ab. Die Plattenemission war so niedrig, dass sie sogar die Anforderungen der entsprechenden japanischen Norm F**** JIS A 5908 erfüllte. Diese Klebstoffe sind wie die UF-Harze farblos.
Similar content being viewed by others
References
Amaral-Labat GA, Pizzi A, Goncalves AR, Celzard A, Rigolet S (2008) Environment-friendly soy flour-based resins without formaldehyde. J Appl Polymer Sci 108:624–632
Ballerini A, Despres A, Pizzi A (2005) Non-toxic, zero-emission tannin-glyoxal adhesives for wood panels. Holz Roh- Werkst 63:477–478
Clariant (France) (2002) Highlink DM: A non toxic agent to produce formaldehyde-free aminoplast cross-linkers. Brochure
Despres A (2006) Mise au point de nouvelles résines aminoplastes écologiques à base de diméthoxyéthanal et sans formaldéhyde pour application en tant que colles pour panneaux de particules. PhD thesis, ENSTIB, University Henri Poincaré – Nancy 1, Epinal, France
Despres A, Pizzi A, Delmotte L (2006) 13C NMR investigation of the reaction in water of UF resins with blocked emulsified isocyanates. J Appl Polymer Sci 99:589–596
El Mansouri N-E, Pizzi A, Salvadó J (2007) Lignin-based wood panel adhesives without formaldehyde. Holz Roh- Werkst 65:65–70
European Norm EN 120 (1995) Wood-based panels. Determination of formaldehyde content. Perforator extraction method
European Norm EN 312 (1995) Wood particleboard – Specifications
Japanese Standards Association (JIS) JIS A 5908 (1994). Particleboards, Tokyo, Japan
Lei H, Pizzi A, Du G (2008) Environment-friendly, mixed tannin/lignin wood resins. J Appl Polymer Sci 107:203–209
Liu Y, Li K (2007) Development and characterization of adhesives from soy protein for bonding wood. Int J Adhesion Adhes 27:59–63
Lorenz L, Frihart CR, Wescott JM (2006) Analysis of soy flour/phenol-formaldehyde adhesives for bonding wood. Proceedings Wood Adhesives 2005, Forest Products Society, Madison, Wisconsin, pp 501–506
Mansouri HM, Pizzi A (2006) Urea-formaldehyde-propionaldehyde physical gelation resins for improved swelling in water. J Appl Polymer Sci 102:5131–5136
Pichelin F, Nakatani M, Pizzi A, Wieland S, Despres A, Rigolet S (2006) Thick wood panels bonded industrially with formaldehyde free tannin adhesives. Forest Prod J 56:31–36
Pizzi A (1983) Wood adhesives: chemistry and technology. M. Dekker, New York
Pizzi A (1994) Advanced wood adhesives technology. M. Dekker, New York
Pizzi A, Meikleham N, Dombo D, Roll W (1995) Autocondensation-based, zero-emission, tannin adhesives for particleboard. Holz Roh- Werkst 53:201–204
Pizzi A, Valenzuela J, Westermeyer C (1993) Non-emulsifiables, water-based, diisocyanate adhesives for exterior plywood, Part 2: industrial application. Holzforschung 47:69–72
Pizzi A, Walton T (1992) Non-emulsifiable, water-based diisocyanate adhesives for exterior plywood, Part 1. Holzforschung 46:541–547
Wang S, Pizzi A (1997) Succinaldehyde-induced water resistance improvements of UF wood adhesives. Holz Roh- Werkst 55:9–12
Wescott JM, Traska A, Frihart CR, Lorenz L (2006) Durable soy-based adhesive dispersions, Proceedings Wood Adhesives 2005, Forest Products Society, Madison, Wisconsin, pp. 263–370
Wieland S, Pizzi A, Hill S, Grigsby W, Pichelin F (2006) The reaction in water of UF resins with isocyanates at short curing times: a 13C NMR investigation. J Appl Polymer Sci 100:1624–1632
Zanetti M, Pizzi A (2004) Dependance on the adhesive formulation of the upgrading of MUF particleboard adhesives and decrease of melamine content by buffer and additives. Holz Roh- Werkst 62:445–451
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Despres, A., Pizzi, A., Vu, C. et al. Colourless formaldehyde-free urea resin adhesives for wood panels . Eur. J. Wood Prod. 68, 13–20 (2010). https://doi.org/10.1007/s00107-009-0344-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00107-009-0344-y