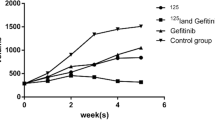
Abstract
Purpose
Development of a safe and effective systemic chemotherapeutic agent for concurrent administration with definitive thoracic radiotherapy remains a major goal of lung cancer management. The synergistic effect of PEGylated liposomal doxorubicin and irradiation was evaluated in lung cancer cell lines both in vitro and in vivo.
Methods
In vitro radiosensitization of A549 and LLC cell lines was evaluated by colony formation assay, γH2AX fluorescent staining and western blot assay, and annexin V staining. A radiosensitization study with healthy human lung-derived cell line BEAS-2B was performed for comparative purposes. In vivo radiosensitization was evaluated by tumor ectopic growth, cell survival, pharmacokinetics, and biodistribution analyses. Cleaved caspase‑3, the marker for apoptosis, was assessed immunohistochemically in A549 xenograft tumors.
Results
Treatment with PEGylated liposomal doxorubicin decreased A549 and LLC cell proliferation in a dose-dependent manner. In vitro studies revealed comparable radiosensitizer advantages of PEGylated liposomal doxorubicin and free doxorubicin, showing equivalent DNA double-strand breaks according to γH2AX fluorescent staining and western blot assays, similar numbers of apoptotic cells in the annexin‑V staining assay, and moderately decreased clonogenic survival. In vivo studies demonstrated markedly slow ectopic tumor growth with prolonged survival following treatment with PEGylated liposomal doxorubicin plus irradiation in both A549 and LLC mouse models, suggesting that PEGylated liposomal doxorubicin is more effective as a radiosensitizer than free doxorubicin in vivo. Pharmacokinetics evaluation showed a longer half-life of approximately 40 h for PEGylated liposomal doxorubicin, confirming that the liposomal carrier achieved controlled release. Biodistribution evaluation of PEGylated liposomal doxorubicin confirmed high accumulation of doxorubicin in tumors, indicating the promising drug delivery attributes of PEGylated liposomal doxorubicin. Although free doxorubicin caused histopathologic myocarditis with the cardiac muscle fibers showing varying degrees of damage, PEGylated liposomal doxorubicin caused no such effects. The immunohistochemical expression of cleaved caspase-3-positive cells was greatest expressed in the irradiation and PEGylated liposomal doxorubicin combined treatment group, indicating prolonged tumoricidal effects.
Conclusions
Our study provides preclinical in vitro and in vivo evidence of the effectiveness of PEGylated liposomal doxorubicin as a radiosensitizer, supporting its potential clinical development as a component of chemoradiotherapy.





Similar content being viewed by others
References
Citrin DE (2017) Recent developments in radiotherapy. N Engl J Med 377:1065–1075. https://doi.org/10.1056/NEJMra1608986
Tyldesley S, Delaney G, Foroudi F, Barbera L, Kerba M, Mackillop W (2011) Estimating the need for radiotherapy for patients with prostate, breast, and lung cancers: verification of model estimates of need with radiotherapy utilization data from British Columbia. Int J Radiat Oncol Biol Phys 79:1507–1515. https://doi.org/10.1016/j.ijrobp.2009.12.070
Chen JL, Tsai YC, Tsai MH et al (2017) Prominin-1-specific binding peptide-modified apoferritin nanoparticle carrying irinotecan as a novel radiosensitizer for colorectal cancer stem-like cells. Part Part Syst Charact 34:1600424. https://doi.org/10.1002/ppsc.201600424
Huang YS, Chen JL, Chen JY et al (2019) Predicting tumor responses and patient survival in chemoradiotherapy-treated patients with non-small-cell lung cancer using dynamic contrast-enhanced integrated magnetic resonance-positron-emission tomography. Strahlenther Onkol 195:707–718. https://doi.org/10.1007/s00066-018-1418-8
Warner A, Dahele M, Hu B et al (2016) Factors associated with early mortality in patients treated with concurrent chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 94:612–620. https://doi.org/10.1016/j.ijrobp.2015.11.030
Tang N, Du G, Wang N, Liu C, Hang H, Liang W (2007) Improving penetration in tumors with nanoassemblies of phospholipids and doxorubicin. J Natl Cancer Inst 99:1004–1015. https://doi.org/10.1093/jnci/djm027
Nesseler JP, Salleron J, Rios M et al (2017) A retrospective cohort study to assess adjuvant concurrent chemoradiation (CCRT) compared to adjuvant radiation therapy (RT) in the treatment of grade 2 and 3 extremity soft tissue sarcomas. Radiother Oncol 125:160–167. https://doi.org/10.1016/j.radonc.2017.08.037
Rehammar JC, Jensen MB, McGale P et al (2017) Risk of heart disease in relation to radiotherapy and chemotherapy with anthracyclines among 19,464 breast cancer patients in Denmark, 1977–2005. Radiother Oncol 123:299–305. https://doi.org/10.1016/j.radonc.2017.03.012
Cui J, Li C, Guo W et al (2007) Direct comparison of two pegylated liposomal doxorubicin formulations: Is AUC predictive for toxicity and efficacy? J Control Release 118:204–215. https://doi.org/10.1016/j.jconrel.2006.12.002
Gabizon AA (2001) Pegylated liposomal doxorubicin: metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest 19:424–436. https://doi.org/10.1081/cnv-100103136
Jiang W, Lionberger R, Yu LX (2011) In vitro and in vivo characterizations of PEGylated liposomal doxorubicin. Bioanalysis 3:333–344. https://doi.org/10.4155/bio.10.204
Bavli Y, Winkler I, Chen BM et al (2019) Doxebo (doxorubicin-free doxil-like liposomes) is safe to use as a pre-treatment to prevent infusion reactions to PEGylated nanodrugs. J Control Release 306:138–148. https://doi.org/10.1016/j.jconrel.2019.06.007
Shieh MJ, Hsu CY, Huang LY, Chen HY, Huang FH, Lai PS (2011) Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells. J Control Release 152:418–425. https://doi.org/10.1016/j.jconrel.2011.03.017
Chou HH, Wang KL, Chen CA et al (2006) Pegylated liposomal doxorubicin (Lipo-Dox) for platinum-resistant or refractory epithelial ovarian carcinoma: a Taiwanese gynecologic oncology group study with long-term follow-up. Gynecol Oncol 101:423–428. https://doi.org/10.1016/j.ygyno.2005.10.027
Kim DW, Blackwell K, Vujaskovic Z et al (2008) A Phase I/II study of neoadjuvant liposomal doxorubicin, paclitaxel and hyperthermia in locally advanced breast cancer. Int J Radiat Oncol Biol Phys 72:S183–S184. https://doi.org/10.1016/j.ijrobp.2008.06.751
O’Connor BM, Lewis SA, Borys N (2010) Initial results of a phase I/II study evaluating the maximum tolerated dose, pharmacokinetics, safety, and efficacy of hyperthermia and lyso-thermosensitive liposomal (LTSL) doxorubicin in patients with breast cancer recurrence at the chest wall (RCW). Int J Radiat Oncol Biol Phys 78:S254–S245. https://doi.org/10.1016/j.ijrobp.2010.07.608
Hagtvet E, Roe K, Olsen DR (2011) Liposomal doxorubicin improves radiotherapy response in hypoxic prostate cancer xenografts. Radiat Oncol 6:135. https://doi.org/10.1186/1748-717X-6-135
Koukourakis MI, Koukouraki S, Giatromanolaki A et al (1999) Liposomal doxorubicin and conventionally fractionated radiotherapy in the treatment of locally advanced non-small-cell lung cancer and head and neck cancer. J Clin Oncol 17:3512–3521. https://doi.org/10.1200/JCO.1999.17.11.3512
Koukourakis MI, Koukouraki S, Giatromanolaki A et al (2000) High intratumoral accumulation of stealth liposomal doxorubicin in sarcomas—rationale for combination with radiotherapy. Acta Oncol 3:207–211. https://doi.org/10.1080/028418600430789
Chen JL, Pan CK, Huang YS et al (2021) Evaluation of antitumor immunity by a combination treatment of high-dose irradiation, anti-PDL1, and anti-angiogenic therapy in murine lung tumors. Cancer Immunol Immunother 70:391–404. https://doi.org/10.1007/s00262-020-02690-w
Chen JL, Chen JP, Huang YS et al (2016) Radiosensitization in esophageal squamous cell carcinoma: effect of polo-like kinase 1 inhibition. Strahlenther Onkol 192:260–268. https://doi.org/10.1007/s00066-016-0951-6
Gyöngyösi M, Lukovic D, Zlabinger K et al (2020) Liposomal doxorubicin attenuates cardiotoxicity via induction of interferon-related DNA damage resistance. Cardiovasc Res 116:970–982. https://doi.org/10.1093/cvr/cvz192
Solazzo SA, Ahmed M, Schor-Bardach R et al (2010) Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology 255:62–74. https://doi.org/10.1148/radiol.09091196
Alibolandi M, Abnous K, Mohammadi M et al (2017) Extensive preclinical investigation of polymersomal formulation of doxorubicin versus doxil-mimic formulation. J Control Release 264:228–236. https://doi.org/10.1016/j.jconrel.2017.08.030
Dunne AL, Price ME, Mothersill C, McKeown SR, Robson T, Hirst DG (2003) Relationship between clonogenic radiosensitivity, radiation-induced apoptosis and DNA damage/repair in human colon cancer cells. Br J Cancer 89:2277–2283. https://doi.org/10.1038/sj.bjc.6601427
Eriksson D, Stigbrand T (2010) Radiation-induced cell death mechanisms. Tumor Biol 31:363–372. https://doi.org/10.1007/s13277-010-0042-8
Ruggeri BA, Camp F, Miknyoczki S (2014) Animal models of disease: pre-clinical animal models of cancer and their applications and utility in drug discovery. Biochem Pharmacol 87:150–161. https://doi.org/10.1016/j.bcp.2013.06.020
Jung J (2014) Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicol Res 30:1–5. https://doi.org/10.5487/TR.2014.30.1.001
Talmadge JE, Singh RK, Fidler IJ, Raz A (2007) Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am J Pathol 170:793–804. https://doi.org/10.2353/ajpath.2007.060929
Zhang W, Fan W, Rachagani S et al (2019) Comparative study of subcutaneous and orthotopic mouse models of prostate cancer: vascular perfusion, vasculature density, hypoxic burden and BB2r-targeting efficacy. Sci Rep 9:11117. https://doi.org/10.1038/s41598-019-47308-z
Sia J, Szmyd R, Hau E (2020) Molecular mechanisms of radiation-induced cancer cell death: a primer. Front Cell Dev Biol 8:41. https://doi.org/10.3389/fcell.2020.00041
Gaillard PJ, Appeldoorn CC, Dorland R et al (2014) Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS One 9:e82331. https://doi.org/10.1371/journal.pone.0082331
Anders CK, Adamo B, Karginova O et al (2013) Pharmacokinetics and efficacy of PEGylated liposomal doxorubicin in an intracranial model of breast cancer. PLoS One 8:e61359. https://doi.org/10.1371/journal.pone.0061359
Tsoutsou PG, Froudarakis ME, Bouros D, Koukourakis MI (2008) Hypofractionated/accelerated radiotherapy with cytoprotection (HypoARC) combined with vinorelbine and liposomal doxorubicin for locally advanced non-small cell lung cancer (NSCLC). Anticancer Res 28:1349–1354
Schiffelers RM, Koning GA, ten Hagen TL et al (2003) Anti-tumor efficacy of tumor vasculature-targeted liposomal doxorubicin. J Control Release 91:115–122. https://doi.org/10.1016/s0168-3659(03)00240-2
Biswas S, Deshpande PP, Perche F, Dodwadkar NS, Sane SD, Torchilin VP (2013) Octa-arginine-modified pegylated liposomal doxorubicin: an effective treatment strategy for non-small cell lung cancer. Cancer Lett 335:191–200. https://doi.org/10.1016/j.canlet.2013.02.020
Acknowledgements
We would like to thank the staff of the Core Lab at the Department of Medical Research, National Taiwan University Hospital, for their technical support.
Funding
This work was supported by the National Taiwan University Hospital (grant number NTUH 109-N4547) and the Ministry of Science and Technology (MST, Taiwan, Contract No. 108-2314-B-002-035-MY2 and No. 109-2314-B-002-094-MY2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
JC, CP, CT, YH, and YL performed the experiments. JC, CP, FH, and WY analyzed and interpreted the data. JC, CP, YH, and YL were major contributors in writing the manuscript. SK and MS supervised the study, reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
J.L.-Y. Chen, C.-K. Pan, Y.-L. Lin, C.-Y. Tsai, Y.-S. Huang, W.-C. Yang, F.-M. Hsu, S.-H. Kuo and M.-J. Shieh declare that they have no competing interests.
Ethical standards
All in vivo experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC 20180464). Humane endpoints were determined according to a clinical scoring system based on that outlined by the IACUC of our institution.
Supplementary Information
66_2021_1835_MOESM1_ESM.pdf
Supplementary Figure 1. Tumor cell viability was evaluated using Cell Counting kit-8 (Sigma-Aldrich, Gillingham, UK). Treatment with pegylated liposomal doxorubicin decreases cell proliferation in a dose-dependent manner.
66_2021_1835_MOESM2_ESM.pdf
Supplementary Figure 2. BALB/c athymic nude mice bearing established subcutaneous A549 cells (mean starting tumor volume = 30 mm3) and C57BL/6 mice bearing established subcutaneous LLC tumors (mean starting tumor volume = 40 mm3) were randomized into five treatment groups and a control group. PEGylated liposomal doxorubicin enhanced tumor suppressive activity and prolonged survivals in both A549 and LLC ectopic mouse models. Mice bearing A549 ectopic tumors were sacrificed on day 45. The immunohistochemically stained cleaved caspase-3 intensity of ectopic tumors was scored using the threshold method in ImageJ and based on three slides per group.
Rights and permissions
About this article
Cite this article
Chen, J.LY., Pan, CK., Lin, YL. et al. Preclinical evaluation of PEGylated liposomal doxorubicin as an effective radiosensitizer in chemoradiotherapy for lung cancer. Strahlenther Onkol 197, 1131–1142 (2021). https://doi.org/10.1007/s00066-021-01835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-021-01835-9