Abstract
Purpose
To assess acute cardiac toxicity caused by intraoperative radiotherapy (IORT) with low-energy x‑rays for early breast cancer.
Methods
We prospectively analyzed pre- and postoperative troponin I and NT-proBNP in 94 women who underwent breast-conserving surgery between 2013 and 2017 at the Department of Gynecology and Obstetrics of the University Medical Center Mannheim, Germany. Thirty-nine women received IORT using low-energy x‑rays during breast-conserving surgery while 55 patients without IORT formed the control group. Demographic and surgical parameters as well as cardiac markers were evaluated.
Results
There were no significant differences concerning age and side of breast cancer between the groups. Furthermore, no significant difference between the troponin I assays of the IORT and control groups could be found (preoperatively: 0.017 ± 0.006 ng/ml vs. 0.018 ± 0.008 ng/ml; p = 0.5105; postoperatively: 0.019 ± 0.012 ng/ml vs. 0.018 ± 0.010 ng/ml; p = 0.6225). N‑terminal fragment of B‑type natriuretic peptide (NT-proBNP) was significantly higher in the control group 24 h after surgery (preoperatively: 158.154 ± 169.427 pg/ml vs. 162.109 ± 147.343 pg/ml; p = 0.56; postoperatively: 168.846 ± 160.227 pg/ml vs. 232.527 ± 188.957 pg/ml; p = 0.0279).
Conclusion
Troponin I levels as a marker of acute cardiac toxicity did not show any significant differences in patients who received IORT during breast-conserving surgery compared to those who did not.
Similar content being viewed by others
Introduction
With a lifetime risk of almost 10%, breast cancer is the most common malignant tumor in the female population worldwide. Early breast cancer treatment in the majority of cases consists of breast-conserving surgery (BCS), which is typically combined with axillary sentinel lymph node biopsy (SNB) followed by external beam whole-breast radiotherapy (EBRT). Other crucial therapeutic principles in the treatment of breast cancer are chemotherapy, endocrine, and targeted therapies.
Over recent years radiotherapy techniques have improved in terms of sophistication and versatility and a decrease in the risk of local relapse was attained by applying an additional tumor bed boost of 10–20 Gy in high-risk patients [1]. A problem of the external application of the tumor bed boost application lies in the potential risk of missing the target, which is considered to be 20–90% [1]. This risk of missing the tumor bed can be reduced by the application of intraoperative radiotherapy (IORT) to the tumor bed during BCS immediately after removal of the tumor [2]. Besides minimizing the risk of “geographical miss,” IORT can shorten the interval between tumor excision and the beginning of adjuvant radiotherapy, thereby making a “temporal miss” unlikely as well [2].
Depending on the technique used as well as the indication, IORT can be applied as a tumor bed boost followed by EBRT (in high-risk patients) or as a standalone therapeutic modality in the sense of accelerated partial breast irradiation (in low-risk patients) [3,4,5].
The mobile Intrabeam® (Carl Zeiss Surgical, Oberkochen, Germany) device is a miniature x‑ray source with a maximum of 50 kV which has been used for IORT during BCS in selected patients at the University Medical Center Mannheim since February 2002 [5,6,7,8]. Thus far it seems that in early low-risk breast cancer settings IORT is non-inferior to EBRT when it comes to local cancer recurrence rates and breast cancer-related mortality [5, 9]. However, in the TARGIT‑A trial, non-inferiority was only shown in the pre-pathology cohort and patients received additional EBRT as indicated [9]. Also, IORT using Intrabeam has been reported to deliver significantly less radiation to normal tissues when compared to EBRT [5,6,7, 10, 11]. Woolf et al. demonstrated that the radiation exposure (using gamma-H2AX in circulating lymphocytes as a biological marker of radiation dose) to the intrathoracic organs (including heart and intrathoracic great vessels) is significantly lower with IORT compared to EBRT [10, 11]. Moreover, in a previous study by our group, postoperative complications were demonstrated to be rare and immediate toxicity was low [12].
Adverse cardiac effects
Cardiac toxicity is one of the most feared complications associated with radiotherapy. Subclinical cardiac damage occurs in >50% of breast cancer survivors treated with radiation therapy [13]. It is known to be mostly a late-onset adverse effect of radiotherapy and might clinically manifest even decades after the original treatment. Its significance is caused foremost by its irreversibility and the quality of life forfeit [14]. Exposure of the heart to ionizing radiation during radiotherapy for breast cancer increases the subsequent rate of ischemic heart disease. The increase is proportional to the mean dose to the heart. Previous studies have shown that the rate of major coronary events increases by 7.4% for each elevated Gy in the mean radiation dose delivered to the heart [15].
In left-sided breast cancer patients EBRT has been shown to raise cardiovascular mortality and morbidity compared to right-sided breast cancer patients [14].
Cardiotoxicity risk is increased by the following individual risk factors: age, smoking, hypercholesterolemia, weight, hypertension, diabetes, family history, and additional oncologic treatments such as targeted, endocrine, and chemotherapies. Hooning et al. showed a significant negative correlation between cardiopathy risk and age at radiotherapy in breast cancer patients [16]. Patients below 35 years of age seem to be especially vulnerable to radiation-induced cardiopathy [16].
Cardiotoxicity after IORT is assumed to be lower than after EBRT, as IORT delivers the lowest maximum dose to the heart in comparison to EBRT [17].
Cardiac markers
Cardiac markers play an important role not only in the diagnosis and assessment of cardiac diseases, but also concerning decision-making and risk stratification. Cardiac troponin T (cTnT) and troponin I (cTnI) are cardiac regulatory proteins that control the calcium-mediated interaction between actin and myosin. While cardiac troponin T is expressed to a small extent in skeletal muscle, cTnI has not been identified outside the myocardium [18]. Cardiac troponins are established biomarkers of acute myocardial injury with very high sensitivity, and are independently predictive of adverse outcomes following noncardiac surgery [19].
A previous study of Skyttä et al. demonstrated that high-sensitivity cardiac troponin T (hscTnT) levels increased during whole-breast adjuvant radiotherapy (RT) in every fifth patient. Moreover, the increase in hscTnT release was positively associated with cardiac radiation doses and with minor changes in left ventricle diastolic function, suggesting that RT caused subclinical myocardial damage [20].
B‑type natriuretic peptide (BNP) is a biomarker that is synthesized and secreted in ventricular myocytes and, to a lesser extent, in the atrial myocardium [19]. It is synthesized as a 108-amino acid prohormone (proBNP) which is cleaved to the 32-residue BNP and the 76-residue N‑terminal fragment of proBNP (NT-proBNP) [21]. The main stimulus for the release of BNP is increased myocardial wall stretch mediated by pressure or volume loading [19].
BNP protects the heart from adverse consequences of overload by increasing natriuresis and diuresis, relaxing vascular smooth muscle, inhibiting the renin–angiotensin–aldosterone system, and by counteracting cardiac hypertrophy and fibrosis [21]. It plays an important role in the diagnosis of congestive heart failure, severity stratification, and therapy control.
In a previous study, patients with left-sided breast cancer showed higher values of NT-proBNP 5 to 22 months after RT when compared with non-RT-treated matched patients, increasing in correlation with high doses in small volumes of heart and ventricle [22].
Jingu et al. could show higher BNP concentrations after radiotherapy of esophageal cancer [23]. Nellesson et al. found an increase in both troponin I and BNP levels in their weekly serum controls during RT in 23 patients receiving RT because of either breast or lung cancer [24].
Hence, BNP concentration might be an early indicator of radiation-induced myocardial damage [24].
The aim of this study was to assess acute cardiotoxicity caused by IORT with low-energy x‑rays using the biomarkers NT-proBNP and troponin I as indicators.
Materials and methods
This study was designed as monocentric and prospective proof of principle study. Eligible for enrollment in the study were all women undergoing breast-conserving surgery who had confirmed invasive breast cancer and were capable of informed consent.
The primary endpoint of the study was to prove that cardiac impact within IORT as an integral part of breast-conserving surgery does not trigger acute cardiac toxicity. To this end, the heart parameters troponin I and NT-proBNP were measured prior to and after breast-conserving surgery in patients with and without IORT.
The eligibility criteria for IORT are multimodal. The decisive criteria included tumor size, tumor–skin distance, intraoperative situs, and patient’s motivation/consent for IORT.
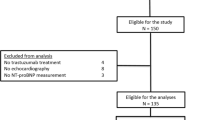
Between 2013 and 2017, a total of 94 women with early breast cancer were included in this study. The local ethics committee approved the protocol and all participants signed informed consent prior to study enrollment. Thirty-nine of the included patients were treated by IORT using the mobile Intrabeam® device (Carl Zeiss Surgical, Oberkochen, Germany) during BCS at the University Medical Center Mannheim, Germany. This system consists of a miniaturized linear accelerator emitting low-energy x‑ray photons (maximum 50 kV), a floor stand and a control unit. The control group consisted of 55 women who did not receive IORT during BCS. The surgical and radiotherapeutic procedures were performed in accordance with hospital and national protocols.
Troponin I and NT-proBNP levels were measured before and 24 h after surgery in all patients. All data were collected in an Excel™ (Microsoft Corporation, Redmond, Seattle, WA, USA) datasheet. After a thorough check for faulty entries, the data were transferred for statistical analysis. All computations were performed using the SAS®, version 9.4, statistics software (SAS Institute, Cary, NC, USA).
Pre- and post-surgery leukocyte counts, hemoglobin, troponin I, and NT-proBNP values were compared using a Mann–Whitney U-test. The same test was used in an attempt to detect between-group differences in the absolute changes (from before surgery to 24 h after surgery) in leukocyte counts and hemoglobin, troponin I and NT-proBNP values. The chi-square test or Fisher’s test was performed to compare the groups in terms of breast cancer characteristics (not including exact tumor size) and location and previous history of cardiovascular diseases. The mean patient age, tumor size, and surgery duration were compared using a t-test.
Results
The data of interest to the investigators were available for all the enrolled patients except for the missing postoperative leukocyte count and hemoglobin level datapoints for 5 patients in the non-IORT group.
There were no significant differences in age (p = 0.4579), overall cardiovascular disease history (p = 0.8901), smoking status (p = 0.5068), and axillary surgical management (p = 0.8732) between the two subgroups as shown in Table 1. Statistically nonsignificant differences were observed in the frequency of antecedent cardiovascular disease barring arterial hypertension (10 patients in the non-IORT vs. 1 in the IORT group; p = 0.075). The location of the breast cancer lesions was comparable in women with and without IORT (Table 2) while the duration of surgery was significantly longer (p < 0.0001) in the IORT group (Table 1). Two patients from the IORT group received neoadjuvant systemic therapy with epirubicin and cyclophosphamide followed by a taxane, and one patient from the control group underwent neoadjuvant treatment with epirubicin and cyclophosphamide followed by paclitaxel enhanced by dual HER2-blockade with trastuzumab and pertuzumab (Table 2).
There were no differences concerning the incidence of multicentric breast cancer upon comparing the two groups (IORT n = 3 [7.7%] vs. control n = 6 [10.9%]; p = 0.7310), whereas tumor size showed significant differences (p = 0.0117) as depicted in Table 2. Furthermore, both groups lacked significant differences in terms of hormonal immunohistochemical characteristics, as depicted in Table 2.
Troponin I levels were not significantly different between the groups in the preoperative (p = 0.5105) or the postoperative (p = 0.6225) setting, as displayed in Table 3. Also, no differences were observed in the troponin I value dynamics between the groups (p = 0.2882). The same is true when comparing the left-sided and right-sided breast cancer patients (Table 3). There was a significant difference in the postoperative NT-proBNP levels, with a significantly lower postoperative NT-proBNP in the IORT group (168.846 ± 160.227 in the IORT-group vs. 232.527 ± 188.957 in the control group; p = 0.0279). Similarly, a significant difference in the absolute change in the NT-proBNP levels (from pre-surgery to post-surgery) was observed between the groups (10.692 ± 67.130 in the IORT group vs. 70.418 ± 126.080 in the control group; p = 0.0044). A subgroup analysis regarding laterality of the breast cancer showed significant NT-proBNP changes only among left-sided IORT and control group patients (p = 0.0365; Table 3).
However, preoperative NT-proBNP levels were similar (158.154 ± 169.427 in the IORT group vs. 162.109 ± 147.343 in the control group; p = 0.5597; Table 3).
The WBC counts as well as the hemoglobin levels and their dynamics (pre- versus postoperative) showed no significant discrepancies between groups (Table 3).
For the purpose of better visualization, waterfall plots depicting the dynamics of cardiac markers of every individual patient were included (Figs. 1 and 2), as well as a comparison of the dynamics of the cardiac markers depicting the IORT group alongside the control group (Figs. 3 and 4).
Discussion
Postoperative radiotherapy is standard of care for patients with early breast cancer and has led to a decrease in cancer recurrence and mortality rates [25]. However, it is not without risks and its unintentional cardiotoxic effects have been observed time and again [26]. The effect of radiation on the heart predictably depends on the percentage of the heart volume that is irradiated and on the cumulative dose of radiation [15, 27]. The leading theory is that myocardial damage is actually a consequence of the indirect effects of a compromised cardiac vasculature [28].
Darby et al. elegantly demonstrated that there is a direct linear correlation between the mean radiation dose and the percentage increase in the rate of major coronary events in the years following EBRT [15]. The cumulative risk of cardiac death rises nonlinearly in the course of time, but the highest percentage of major coronary events was recorded during the first 10 years after radiotherapy.
The distance between the heart and the radiation source as well as the characteristics of the source itself determine the percentage of the myocardium affected by the radiation [17, 29]. As Aziz et al. have demonstrated, the Intrabeam® device emits low-energy x‑rays and has the most favorable penetration profile when compared to EBRT and MammoSite balloon brachytherapy (Proxima Therapeutics, Alpharetta, GA, USA) [17]. However, long-term cardiac toxicity remains to be studied and EBRT is added to the IORT protocol in some high-risk patients [9].
Considering all the facts stated above, one would expect the IORT technique to be significantly less cardiotoxic than those previously employed in the early breast cancer setting. This could possibly also contribute to the circumstance demonstrated by Vaidya et al., who observed a significant increase in non-cancer-related deaths in the EBRT group of the TARGIT trial as compared to the IORT group [5]. Of equal importance is the fact that the local recurrence rates were similar between the groups if there was no delay between surgery and IORT and if patients with high-risk pathologic features received EBRT after IORT.
Although the chronic effects of radiation on the heart are well known, there are a limited number of studies analyzing acute heart damage in patients undergoing EBRT, and the findings of these are ambiguous [28, 30,31,32,33,34,35]. To our knowledge, there has only been a single study assessing IORT in this context [28]. The patients were treated for early breast cancer with the Intrabeam IORT technique. However, in contrast to our enrollment criteria, Saibene et al. excluded patients with preexisting heart disease. Also, no baseline BNP measurements were performed. Nonetheless, the authors conclude that there is no evidence of acute myocardial damage in patients receiving IORT. Finally, it is difficult to compare the cardiotoxic effect of EBRT and IORT in light of this paucity of published data.
Our study also aims to assess acute cardiotoxicity of IORT with low-energy x‑rays in patients with early breast cancer using serial troponin I and NT-proBNP measurements as indicators of radiation-induced acute heart damage. The demographic and surgical parameters of the interventional and the control cohort were observed to be similar, with significant differences only in terms of surgery duration and tumor size (Table 1 and 2). As expected, the patients undergoing IORT within their breast-conserving surgery had spent more time in the operating room (OR) due to the time-consuming intraoperative radiotherapy (p < 0.0001). The smaller tumor size in the IORT group (p = 0.0117) is not surprising and is the consequence of the technical limitations of the Intrabeam® device, which has a maximum applicator size of 5 cm.
Leukocyte counts and hemoglobin concentrations did not differ significantly between the groups, demonstrating the low acute bone marrow suppression potential of IORT. Also, the prolonged surgery in the IORT group was obviously not associated with significant blood loss.
We showed that in our cohort, IORT did not influence pre- or postoperative troponin I levels. Surprisingly, while NT-proBNP levels were similar between the groups before surgery, they were significantly higher in the non-IORT group after surgery. Interestingly, in a further subgroup analysis, this difference turned out to be significant only in the left-sided breast cancer cases, as presented in Table 3. The NT-proBNP statistics are obviously not caused by a single patient’s levels, but a versatile diapason of values, as depicted in Fig. 2. The difference could conceivably be a consequence of the fact that patients with non-hypertensive cardiovascular disease were evidently overrepresented in the non-IORT group. Smoking status and mean age did not differ significantly between the groups and patients in both groups had a similar proportion of left-sided cancers. On the other hand, surgery supplemented by IORT was longer, yet it did not seem to influence NT-proBNP levels negatively.
Our results speak in favor of IORT’s acute cardiac safety and are concordant with the recent findings of Saibene et al. in terms of the absence of a significant troponin I increase associated with IORT [28]. Although we can conclude that IORT doesn’t seem to have an acute cardiotoxic effect, we would not, based on these finding, be able to claim that IORT has a beneficial effect on cardiac health, as there is no pathophysiological explanation for such a result.
Our study is limited by various factors simultaneously affecting cardiac toxicity, foremost the additional systemic therapy regimens consisting of chemotherapy or endocrine and/or targeted therapy and intraoperative narcotic management. Another limitation lies in a relatively small cohort size, despite being the largest trial currently addressing the aforementioned acute cardiac safety of IORT. The limited cohort size in turn causes a lack of statistical power to exclude small differences. Furthermore, the absence of a predefined statistical hypothesis comprises another limitation of our study. Also, the frequency of diabetes mellitus was not analyzed. Since this disease is well known to cause microvascular complications, it could be a confounder that we did not control for in our study. Finally, technical innovations such as DIBH can significantly lower cardiac dose exposure and decrease the risk of cardiac toxicity from EBRT [36, 37].
Conclusion
In conclusion, these findings speak in favor of acute cardiac safety of IORT with low-energy x‑rays within the scope of BCS for early breast cancer. While IORT doesn’t seem to cause acute myocardial damage as inferred by troponin I and NT-proBNP dynamics, further follow-up studies are crucial to investigate the long-term cardiac effects of IORT.
References
Blank E, Kraus-Tiefenbacher U, Welzel G et al (2010) Single-center long-term follow-up after Intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X‑rays. Ann Surg Oncol 17(S3):352–358. https://doi.org/10.1245/s10434-010-1265-z
Herskind C, Steil V, Kraus-Tiefenbacher U, Wenz F (2005) Radiobiological aspects of Intraoperative radiotherapy (IORT) with isotropic low-energy X rays for early-stage breast cancer. Radiat Res 163(2):208–215. https://doi.org/10.1667/rr3292
Ruano-Ravina A, Cantero-Muñoz P, Urién AE (2011) Efficacy and safety of intraoperative radiotherapy in breast cancer: a systematic review. Cancer Lett 313(1):15–25. https://doi.org/10.1016/j.canlet.2011.08.020
Vaidya JS, Joseph DJ, Tobias JS et al (2010) Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT‑A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 376(9735):91–102. https://doi.org/10.1016/s0140-6736(10)60837-9
Vaidya JS, Wenz F, Bulsara M et al (2014) Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5‑year results for local control and overall survival from the TARGIT‑A randomised trial. Lancet 383(9917):603–613. https://doi.org/10.1016/s0140-6736(13)61950-9
Kraus-Tiefenbacher U, Bauer L, Scheda A et al (2006) Long-term toxicity of an intraoperative radiotherapy boost using low energy X‑rays during breast-conserving surgery. Int J Radiat Oncol Biol Phys 66(2):377–381. https://doi.org/10.1016/j.ijrobp.2006.05.042
Neumaier C, Elena S, Grit W et al (2012) TARGIT-E(lderly)—prospective phase II study of intraoperative radiotherapy (IORT) in elderly patients with small breast cancer. BMC Cancer 12:171. https://doi.org/10.1186/1471-2407-12-171
Sedlmayer F, Reitsamer R, Wenz F et al (2017) Intraoperative radiotherapy (IORT) as boost in breast cancer. Radiat Oncol 12(1):23. https://doi.org/10.1186/s13014-016-0749-9
Abo-Madyan Y, Welzel G, Sperk E et al (2019) Single-center long-term results from the randomized phase‑3 TARGIT‑A trial comparing intraoperative and whole-breast radiation therapy for early breast cancer. Strahlenther Onkol 195(7):640–647. https://doi.org/10.1007/s00066-019-01438-5
Woolf DK, Williams NR et al (2014) Biological dosimetry for breast cancer radiotherapy: a comparison of external beam and intraoperative radiotherapy. SpringerPlus 30(3):329. https://doi.org/10.1186/2193-1801-3-329
Williams NR, Pigott KH, Brew-Graves C et al (2014) Intraoperative radiotherapy for breast cancer. Gland Surg 3(2):109–119. https://doi.org/10.3978/j.issn.2227-684X.2014.03.03
Tuschy B, Berlit S, Romero S et al (2013) Clinical aspects of intraoperative radiotherapy in early breast cancer: short-term complications after IORT in women treated with low energy x‑rays. Radiat Oncol. https://doi.org/10.1186/1748-717x-8-95
Hardenbergh PH, Munley MT, Bentel GC et al (2001) Cardiac perfusion changes in patients treated for breast cancer with radiation therapy and doxorubicin: preliminary results. Int J Radiat Oncol Biol Phys 49(4):1023–1028. https://doi.org/10.1016/s0360-3016(00)01531-5
Mege A, Zioueche A, Pourel N et al (2011) Radiation-related heart toxicity. Cancer Radiother 15(6–7):495–503. https://doi.org/10.1016/j.canrad.2011.06.003
Darby SC, Ewertz M, Mcgale P et al (2013) Risk of Ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368(11):987–998. https://doi.org/10.1056/nejmoa1209825
Hooning MJ, Aleman BM, Rosmalen AJV, Kuenen MA, Klijn JG, Leeuwen FEV (2006) Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. Int J Radiat Oncol Biol Phys 64(4):1081–1091. https://doi.org/10.1016/j.ijrobp.2005.10.022
Aziz M, Schneider F, Clausen S et al (2011) Can the risk of secondary cancer induction after breast conserving therapy be reduced using intraoperative radiotherapy (IORT) with low-energy x‑rays? Radiat Oncol 6(1):174. https://doi.org/10.1186/1748-717x-6-174
Sharma S, Jackson PG, Makan J (2004) Cardiac troponins. J Clin Pathol 57(10):1025–1026. https://doi.org/10.1136/jcp.2003.015420
Afshani N, Biccard B, Dyer R (2012) The role of biomarkers and B‑type natriuretic peptide in diagnosis and perioperative risk prediction. South Afr J Anaesth Analg 18(6):303–308. https://doi.org/10.1080/22201173.2012.10872870
Skyttä T, Tuohinen S, Boman E, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen P‑L (2015) Troponin T‑release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol. https://doi.org/10.1186/s13014-015-0436-2
Vuolteenaho O, Ala-Kopsala M, Ruskoaho H (2005) BNP as A Biomarker in Heart Disease. Adv Clin Chem. https://doi.org/10.1016/s0065-2423(05)40001-3
D’Errico MP, Grimaldi L, Petruzzelli MF et al (2012) N‑terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys. https://doi.org/10.1016/j.ijrobp.2011.03.058
Jingu K, Nemoto K, Kaneta T et al (2007) Temporal change in brain natriuretic peptide after radiotherapy for thoracic esophageal cancer. Int J Radiat Oncol Biol Phys 69(5):1417–1423. https://doi.org/10.1016/j.ijrobp.2007.05.054
Nellessen U, Zingel M, Hecker H, Bahnsen J, Borschke D (2010) Effects of radiation therapy on myocardial cell integrity and pump function: which role for cardiac biomarkers? Chemotherapy 56(2):147–152. https://doi.org/10.1159/000313528
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Cuzick J, Stewart H, Rutqvist L et al (1994) Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 12(3):447–453. https://doi.org/10.1200/JCO.1994.12.3.447
Taylor CW, Brønnum D, Darby SC et al (2011) Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol 100(2):176–183. https://doi.org/10.1016/j.radonc.2011.01.020
Saibene T, Michieletto S, Evangelista L et al (2014) Intraoperative radiotherapy during breast cancer surgery: acute and chronic cardiac safety tested by an ultra-sensitive troponin and N‑terminal Pro-B-type natriuretic peptide. Eur J Oncol 19(3):159–166
Wenz F, Welzel G, Blank E et al (2010) Intraoperative radiotherapy as a boost during breast-conserving surgery using low-kilovoltage X‑rays: the first 5 years of experience with a novel approach. Int J Radiat Oncol Biol Phys 77(5):1309–1314. https://doi.org/10.1016/j.ijrobp.2009.06.085
Marks LB, Yu X, Prosnitz RG et al (2005) The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 63(1):214–223. https://doi.org/10.1016/j.ijrobp.2005.01.029
Eisenman A (2006) Troponin assays for the diagnosis of myocardial infarction and acute coronary syndrome: where do we stand? Expert Rev Cardiovasc Ther 4(4):509–514. https://doi.org/10.1586/14779072.4.4.509
Hughes-Davies L, Sacks D, Rescigno J, Howard S, Harris J (1995) Serum cardiac troponin T levels during treatment of early-stage breast cancer. J Clin Oncol 13(10):2582–2584. https://doi.org/10.1200/jco.1995.13.10.2582
Erven K, Florian A, Slagmolen P et al (2013) Subclinical cardiotoxicity detected by strain rate imaging up to 14 months after breast radiation therapy. Int J Radiat Oncol Biol Phys 85(5):1172–1178. https://doi.org/10.1016/j.ijrobp.2012.09.022
Rodemann H, Bamberg M (1995) Cellular basis of radiation-induced fibrosis. Radiother Oncol 35(2):83–90. https://doi.org/10.1016/0167-8140(95)01540-w
Demissei BG, Freedman G, Feigenberg SJ et al (2019) Early Changes in Cardiovascular Biomarkers with Contemporary Thoracic Radiation Therapy for Breast Cancer, Lung Cancer, and Lymphoma. Int J Radiat Oncol Biol Phys 103(4):851–860. https://doi.org/10.1016/j.ijrobp.2018.11.013
Duma M, Baumann R, Budach W et al (2019) Heart-sparing radiotherapy techniques in breast cancer patients: a recommendation of the breast cancer expert panel of the German society of radiation oncology (DEGRO). Strahlenther Onkol 195:861–871. https://doi.org/10.1007/s00066-019-01495-w
Piroth MD, Baumann R, Budach W et al (2019) Heart toxicity from breast cancer radiotherapy. Strahlenther Onkol 195:1–12. https://doi.org/10.1007/s00066-018-1378-z
Funding
No grants or other forms of financial support were received by any of the authors in relation to this study or the writing of this article.
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: Benjamin Tuschy, Sebastian Berlit, Stefan Stefanovic. Methodology: Elena Sperk, Frederik Trinkmann. Formal analysis and investigation: Christel Weiß, Stefan Stefanovic, Benjamin Tuschy. Writing—original draft preparation: Stefan Stefanovic. Writing—review and editing: Marc Sütterlin, Benjamin Tuschy, Sebastian Berlit. Resources: Marc Sütterlin, Frederik Wenz. Supervision: Marc Sütterlin, Frederik Wenz.
Corresponding author
Ethics declarations
Conflict of interest
S. Stefanovic, S. Berlit, E. Sperk, F. Wenz, C. Weiß, F. Trinkmann, M. Sütterlin, and B. Tuschy declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human biological material were in accordance with the ethical standards of the institutional and/or national research committee—Heidelberg University Ethics Committee II, Medical Faculty Mannheim (2013-578N-MA)—and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stefanovic, S., Berlit, S., Sperk, E. et al. Cardiac serum marker alterations after intraoperative radiotherapy with low-energy x-rays in early breast cancer as an indicator of possible cardiac toxicity. Strahlenther Onkol 197, 39–47 (2021). https://doi.org/10.1007/s00066-020-01671-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01671-3