Abstract
Purpose
This study was conducted to investigate the potential predictive value of tumor budding for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer.
Patients and methods
Surgical specimens of 128 ypUICC (Union for International Cancer Control) stage 0–III mid-to-low rectal cancer patients were identified from a prospectively maintained colorectal cancer database and classified into two groups using the 10 high-power field average method: none/mild tumor budding (BD-0) and moderate/severe tumor budding (BD-1). Overall survival, relapse-free survival (RFS), and recurrence estimates were calculated using the Kaplan–Meier method and compared with the log-rank test. For RFS, a multivariable Cox’s proportional hazards regression analysis was performed.
Results
No (n = 20) or mild (n = 27) tumor budding (BD-0) was identified in 47 (37%) and moderate (n = 52) or severe (n = 29) tumor budding (BD-1) in 81 (63%) surgical specimens. Positive tumor budding (BD-1) was associated with significantly reduced T‑level downstaging (P < 0.001) and tumor regression (P < 0.001). After a median follow-up time of 7 years (range 2.9–146.7 months), BD-0 patients had more favorable 5‑year RFS (90 vs. 71%, P = 0.02) and distant recurrence (2 vs. 12%, P = 0.03) estimates. Multivariable analyses confirmed BD-1 as a negative predictive parameter for RFS (hazard ratio = 3.44, 95% confidence interval 1.23–9.63, P = 0.018).
Conclusions
Our data confirm tumor budding as a strong prognostic factor and its potential predictive value for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer patients. This provides the opportunity to modify and individualize neoadjuvant therapy regimens for non-responders.
Zusammenfassung
Hintergrund und Ziele
Die Studie untersucht die Wertigkeit des „Tumor Budding“ als möglichen prädiktiven Faktor für ein Ansprechen auf die neoadjuvante Radiochemotherapie beim lokal fortgeschrittenen Rektumkarzinom.
Patienten und Methoden
Resektionspräparate von 128 Patienten mit einem lokal fortgeschrittenen Rektumkarzinom im ypUICC (Union for International Cancer Control) Stadium 0–III wurden aus einer prospektiv angelegten Datenbank identifiziert und hinsichtlich „Tumor Budding“ in zwei Gruppen eingeteilt: BD-0 (kein bis mildes „Tumor Budding“) und BD-1 (mäßig bis hochgradiges „Tumor Budding“). Primäre Endpunkte der Studie waren die lokale Kontrolle, die Fernmetastasierung sowie das Gesamt- und das krankheitsfreie Überleben (RFS), die mit der Kaplan-Meier-Methode berechnet und mittels Log-rank-Test auf Signifikanz geprüft wurden. Für das RFS wurde mittels Cox-Regression multivariat getestet.
Ergebnisse
Die retrospektive Analyse der Resektionspräparate ergab ein BD-0 bei 47 (37 %) und ein BD-1 bei 81 Patienten (63 %). Positives „Tumor Budding“ (BD-1) war signifikant mit einer erniedrigten Ansprechrate auf die neoadjuvante Radiochemotherapie sowohl hinsichtlich T‑Level-Downstaging (P < 0,001) als auch Tumorregression (P < 0,001) assoziiert. Dabei zeigten BD-0-Patienten ein signifikant besseres 5‑Jahres-RFS (90 vs. 71 %; P = 0,02) sowie eine bessere 5‑Jahres-Fernmetastasierungsrate (2 vs 12 %; P = 0,03). In der multivariablen Analyse wurde BD-1 als negativ prädiktiver Faktor für das RFS identifiziert (HR = 3,44; 95 %-Konfidenzintervall 1,23–9,63; P = 0,018).
Schlussfolgerung
Unsere Daten bestätigen die Wertigkeit des „Tumor Budding“ als möglichen prädiktiven und prognostischen Faktor für ein Ansprechen auf die neoadjuvante Radiochemotherapie beim lokal fortgeschrittenen Rektumkarzinom. Damit könnte die Bestimmung des „Tumor Budding“ als Stratifikationsparameter für eine individualisierte neoadjuvante Therapie dienen.
Similar content being viewed by others
Introduction
The multidisciplinary approach including radiotherapy, chemotherapy, and surgery is regarded as the standard of care for locally advanced (T3/4 and/or node positive) mid-to-low rectal cancer patients [1, 2]. Neoadjuvant regimens consisting of preoperative long-course 5‑FU (5‑fluorouracil) based chemoradiotherapy (CRT; 45–50.4 Gy, 25–28 fractions) and preoperative short-course radiotherapy (25 Gy, five fractions) are considered the primary treatments of choice [3,4,5]. The CAO/ARO/AIO-94 trial demonstrated the superiority of preoperative CRT with respect to local control, treatment compliance, and overall toxicity profile, but not in overall survival benefit, when compared to postoperative CRT [2, 5]. However, in patients with a complete or near complete pathological response receiving preoperative CRT, there was an improvement in long-term outcomes independent of clinicopathologic parameters [6]. Further randomized controlled trials (SRCT, TME, FFCD9201, EORTC22912, TROG 01.04, and FOWARC) have confirmed the most beneficial impact of preoperative therapy (radiotherapy and/or chemotherapy) on local control [7,8,9,10,11,12,13].
Some aspects of preoperative therapy modalities such as the optimal radiotherapy fractionation, the interval between radiotherapy and surgery, or the inclusion of oxaliplatin are still under debate [13,14,15,16,17,18,19,20,21]. Out of seven randomized controlled studies evaluating the neoadjuvant use of oxaliplatin in the treatment of locally advanced rectal cancer, only two (FORWARC and CAO/ARO/AIO-04) demonstrated a beneficial effect on early endpoints (such as pathological complete response rate) [13, 19]. Furthermore, only the CAO/ARO/AIO-04 study demonstrated a significant improvement in disease-free survival [19].
Irrespective of a reliable tumor downstaging with 15–27% complete responders and stable local recurrence rates of 6%, more than half of the patients show no or just minor response to neoadjuvant therapy and develop distant recurrences in over 25% of cases [18, 22, 23]. Non-responsiveness exposes patients to the risks of toxicity whilst delaying surgery, for no apparent benefit [24]. Pretreatment identification of these patients and implementation of an individualized neoadjuvant therapy regimen may decrease recurrence rates and reduce perioperative complications.
While the classical tumor staging system (TNM) is accepted as the strongest predictor of clinical outcome, it has certain failings in stratifying patient subsets with intermediate tumor response after neoadjuvant therapy into more meaningful prognostic groups [25]. The assessment of tumor regression grading may overcome this shortcoming by measuring the degree of cellular tumor response to neoadjuvant therapy [6, 26, 27]. Both the TNM classification and tumor regression grading are only available in the resected specimen after neoadjuvant therapy and thus are not accessible in pretreatment specimens to assist with the planning of a modified neoadjuvant therapy.
Tumor budding is a promising histomorphological prognostic factor reported in 20–40% of colorectal cancer (CRC) cases [28]. It is defined as the presence of detached isolated single cancer cells or small clusters of up to four cells at the invasive front of epithelial cancers and is associated with lymphovascular invasion, distant metastases, and poor prognosis [28, 29]. Tumor buds in CRC specimens represent the histological phenotype of epithelial-to-mesenchymal transition (EMT) [30, 31]. This transition is characterized by a series of cell alterations (loss of cell adhesion molecules, cytoskeletal alterations, increased production of extracellular matrix components, resistance to apoptosis, and the ability to degrade basement membrane) resulting in a phenotype with increased migratory capacity and invasiveness [32,33,34]. An important factor in this process is that a subset of carcinoma cells acquire the properties of stem cells, promoting long-term tumor propagation, drug resistance, and development of metastases [35, 36].
Some challenges and caveats exist when interpreting the role of tumor budding in neoadjuvantly treated rectal cancer patients, as most of the existing knowledge is derived from colon and historical rectal cancer patients before the era of neoadjuvant therapy regimens. There is, however, a considerable lack of knowledge about the role of tumor budding in neoadjuvantly treated rectal cancer patients, with only six published articles to date in the English literature exploring this topic [24, 29, 37,38,39,40].
The aim of this retrospective cohort study was to examine the impact of tumor budding, analyzed in the resected specimens of 128 neoadjuvantly treated mid-to-low rectal cancer patients, on long-term outcome and to evaluate its potential predictive value for neoadjuvant chemoradiotherapy (nCRT) response.
Materials and methods
Patients
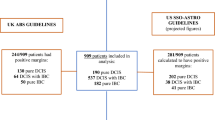
The study cohort was identified from the institutional prospectively maintained colorectal cancer database including a well-characterized and previously published cohort of 144 patients [26]. All patients presented with locally advanced (cT3/4 and/or clinically node positive) mid-to-low rectal cancer who received nCRT prior to total mesorectal (TME) surgery after an interval of 4–6 weeks. Out of these 144 patients, 16 were excluded before analysis because of stage IV disease (n = 12), R2 resections (defined as incomplete local resection; n = 3), and an interval >90 days between end of nCRT and TME surgery (n = 1). The final study cohort comprised a total of 128 consecutive non-metastasized ypUICC (Union for International Cancer Control) stage 0–III rectal cancer patients treated with long-course nCRT and TME surgery between January 2003 and December 2012 at our tertiary care center (Fig. 1). Patients with R1 resection (defined as tumor present 1 mm or less from the radial/resection margin) remained in the study cohort. The resected specimens were assessed retrospectively with a standardized protocol for the presence of tumor budding (described below). Institutional review board approval was obtained to review records and report results.
Neoadjuvant therapy
All patients received long-course nCRT, which consisted of either oral capecitabine or intravenously administered 5‑fluoruracil during 6 weeks of radiotherapy. One-hundred-eleven patients (87%) received a total dose of 45 Gy delivered in three or four high-energy photon beams by a three-dimensional conformal technique, in 25 fractions with daily fractional doses of 1.8 Gy (5 Fx/week) in 5 consecutive weeks. In ten patients (8%), a boost to the macroscopic tumor of up to 50.4 Gy was applied. The remaining six patients received a reduced total dose of 40 to 44 Gy. In approximately half of the patients (52%), oxaliplatin was used as an adjunct to the concomitant chemotherapy. Ten patients (8%) were treated within the ABCSG 95 (Austrian Breast & Colorectal Cancer Study Group) trial protocol (NCT00297141) [41, 42]. Failure to complete nCRT was an exclusion criterion.
Pathologic examination and tumor budding
Pathology specimens were examined independently by three gastrointestinal pathologists (RU, EK, and DN) who were blinded to the patient’s outcome. All rectal specimens were processed macroscopically according to the national guidelines of the Austrian Society of Pathology with details published previously [43, 44]. Completeness of resection was scored as R0 for negative margins (regardless of distance between tumor and resection margins), R1 for microscopic tumor present 1 mm or less from the radial/resection margin, and R2 for gross residual tumor. Pathologic stage (ypT and ypN) was determined according to the 7th AJCC TNM classification [25]. Additionally, the pathological response of the resected specimens to nCRT was graded according the four-category TRG system developed by the American Joint Committee on Cancer and the College of American Pathologists (AJCC/CAP), published elsewhere [26]: in short, complete response (AJCC/CAP grade 0) is characterized by no viable cancer cells, moderate response (AJCC/CAP grade 1) by single or small groups of cancer cells, minimal response (AJCC/CAP grade 2) by residual cancer outgrown by fibrosis, and finally poor response (AJCC/CAP grade 3) by minimal or no tumor cell death with extensive residual cancer in the resected specimen.
Budding focus was defined as the presence of isolated cancer cells or clusters of up to four cancer cells either within the tumor or at the invasive tumor front [45]. As proposed by Koelzer et al. [46], the 10 high-power fields (HPF) method was applied for a quantitative assessment of tumor budding: (i) All H&E slides with tumor areas of the resected specimen were continuously screened for the highest grade of tumor budding at low magnification (× 4 up to × 10). (ii) Based on this histomorphological preselection, tumor buds were then identified by the pathologist and counted in a total of 10 HPFs (× 40; Fig. 2a–d; [47]). Consecutively, the average number of buds in these 10 HPFs was calculated. Tumor budding was categorized as “none” if there was no budding focus (n = 20), as “mild” if there was up to one budding focus (n = 27), as “moderate” if there were more than one and less than five budding foci (n = 52), and as “severe” if more than five budding foci (n = 29) could be observed in the average of 10 HPF (400 × magnification). For further statistical analyses we then formed two groups: BD-0 (none or mild tumor budding; n = 47) and BD-1 (moderate or severe tumor budding; n = 81).
Follow-up and oncologic outcomes
Patient follow-up was performed according to the guidelines of the Austrian Society of Surgical Oncology [48]. Outcome measures assessed in this study included survival estimates (overall [OS] and relapse-free survival [RFS]) and recurrence rates (overall [OR], distant [DR], and local recurrence [LR]). Time-to-event endpoints were calculated from the date of primary surgery. Overall survival was defined as time to death, irrespective of cause, and RFS as the time between surgery and the first recurrence event or death, irrespective of cause. Relapse of disease was defined as pathological, radiological, or clinical determination of rectal cancer recurrence confined to the prior pelvic treatment field (LR) or at any other site, including but not limited to the liver, lungs, and retroperitoneum (DR).
Statistical analysis
Clinicopathological characteristics were compared between the two groups BD-0 and BD-1. Statistical significance in differences of clinicopathological variables were tested with the independent samples t‑test and Mann–Whitney U test for normally and non-normally distributed data, respectively. For the comparison of proportions, the χ2 test or Fisher exact test were used, as appropriate.
For the analyses of the 5‑year survival and recurrence estimates, all events after 60 months of follow-up and patients with a date of last contact more than 60 months after diagnosis were censored at 60 months. Loss to follow-up was assessed with the completeness of follow-up index C as described by Clark et al. [49]. This index quantifies the effect of losses to follow-up as the ratio of the total observed person-time of follow-up as a percentage of the potential time of follow-up. OS, RFS, and recurrence rates were estimated using the Kaplan–Meier method and compared with the log-rank test.
For RFS, a multivariable Cox proportional hazards model was calculated to adjust for baseline differences between BD-0 and BD-1 groups. All the predictors which had a P-value ≤0.10 in the univariate analyses were put in a forward step procedure into the model by keeping variables with a P < 0.05 and excluding those with a P > 0.05. All the tests are two-sided, and a P-value <0.05 indicates a statistically significant difference. Statistical analyses were performed with STATA release 14.2 (StataCorp LLC, College Station, TX).
Results
Patients
The clinical pretreatment characteristics and pathologic outcomes of the tumors in the BD-0 and BD-1 groups are shown in Tables 1 and 2. Between January 2003 and December 2012, 128 patients (87 males) with a mean age of 64 years (range 34–84 years) were identified for this retrospective cohort study. After a median interval of 5.1 weeks (range 2.7–9.3 weeks) from the end of nCRT, a low anterior (72%, 92 of 128) or an abdominoperineal rectal resection with TME (28%, 36 of 128) was performed. Six patients (5%) had positive circumferential resection margin involvement, defined as microscopic evidence of tumor cells 1 mm or less from the margin.
Of the 128 patients, 47.9% (58 of 121) subsequently received adjuvant chemotherapy, consisting of fluoropyrimidine (5-FU/leucovorin or capecitabine) in all patients except one. Sixty-four percent (37 of 58 patients) of those receiving adjuvant chemotherapy additionally received oxaliplatin. The proportion of patients receiving adjuvant chemotherapy did not differ significantly between BD-0 and BD-1 groups (P = 0.35; Fig. 3).
Flow chart of adjuvant chemotherapy in 128 neoadjuvant chemoradiotherapy treated locally advanced rectal cancer patients. * death within 30 days after surgery (n = 2), adjuvant chemotherapy status not available (n = 5), Adj adjuvant, BD tumor budding, CTX chemotherapy, CAPOX capecitabine combined with oxaliplatin, FOLFOX folinic acid combined with 5‑fluorouracil and oxaliplatin, 5‑FU 5‑fluorouracil, OX oxaliplatin
Tumor budding and response
None (n = 20) or mild (n = 27) tumor budding (BD-0) was identified in the specimens of 47 (37%) patients and moderate (n = 52) or severe (n = 29) tumor budding (BD-1) in 81 patients (63%). There were 43 patients (33.6%) with no downstaging effect (T-level or nodal downstaging). Positive tumor budding (BD-1) was associated with significantly reduced T‑level downstaging (P < 0.001) and tumor regression as assessed by the four-tier AJCC/CAP tumor regression grading system (P < 0.001) [50]. With regards to tumor regression, a complete response with no residual viable tumor cells was achieved in 16 patients (13%) and a moderate response in 39 patients (30%), with only a single or small groups of cancer cells remaining. Nevertheless, over half of the patients had either minimal (42%, 54 of 128) or poor response (15%, 19 of 128).
Patients with BD-1 had a statistically significant association with AJCC/CAP tumor regression grade (P < 0.001), ypT (P < 0.001), ypN (P = 0.01), nodal involvement (P = 0.02), lymphatic invasion (P = 0.003), and ypUICC stage (P < 0.001; Table 2).
Follow-up and events
All patients were followed at least for 5 years or to the date of death or loss to follow-up. A complete follow-up was achieved in 83% of patients as assessed by the completeness of follow-up index C as described by Clark et al. [49]. During the median follow-up time of 7 years (range 2.9–146.7 months), twenty patients (15.6%; BD-0: n = 4 and BD-1: n = 16) died. Of these, six patients (30%; BD-0: n = 1 and BD-1: n = 5) died within the first year. Overall tumor recurrence was diagnosed in 15 of the 128 patients (11.7%; BD-0: n = 2 and BD-1: n = 13). Locoregional recurrence occurred in six (4.7%), distant metastasis in twelve (9.4%), and combined locoregional and distant recurrence in three patients (2.3%).
Time to event analyses
BD-1 patients had a considerably poorer 5‑year RFS, OR, and DR rates compared to BD-0 patients (RFS: 71% vs. 90%; P = 0.02; OR: 16% vs. 2%; P = 0.04; and DR: 12% vs. 2%; P = 0.03, respectively; Fig. 4a–c, Table 3). In univariate analyses, several variables, i.e., tumor budding, ASA classification, secondary malignancies, age, ypT, and circumferential resection margin, were significantly associated (P < 0.05) with RFS (Table 4). In multivariate analysis, only tumor budding, ASA classification, and circumferential resection margin were independently prognostic for RFS (Table 5). The results from this study failed to demonstrate a statistically significant association between BD-1 and OS and LR (P = 0.09 and P = 0.13, respectively).
Discussion
Tumor budding has been described most extensively in early and advanced colorectal cancer, implicating several scenarios in which this morphological feature might influence clinical decision making [33]. First, tumor budding could serve as a predictor of lymph node metastases in malignant polyps, which would suggest the need for resection. Second, budding may be indicative of tumor progression in stage II colorectal cancer to stratify for adjuvant therapy. Finally, tumor budding could be relevant as a predictor of tumor response to neoadjuvant therapy in pretreatment biopsies of locally advanced rectal cancer specimens [45, 46].
The first step in the development of a tumor bud seems to be its detachment from the main tumor body by loss of the adhesion molecule E‑cadherin [51]. Overall, EMT is implicated in the underlying molecular mechanism of budding, which includes loss of E‑cadherin in addition to the expression of fibronectin in the cytoplasm. These changes are suggestive of a more mesenchymal phenotype and also suggest a more aggressive tumor bud [51, 52]. Dysregulation of cell stemness phenotype (e.g., β‑catenin and CD133), cell–cell interaction (e.g., CD44 and E‑cadherin), and cell–matrix interaction (e.g., matrix metalloproteinase) as well as of inflammation (e.g., cytotoxic T cells) are essentially involved in tumor budding [53]. Analysis of these markers in pretreatment (and rarely post-pretreatment) specimens of colorectal cancer suggests that they could be linked to radio-chemoresistance via selective EMT properties [24, 54, 55]. Based on observations of existing precursors, it has been hypothesized that the EMT process is initiated through alterations induced by radiotherapy [24, 56]. It remains unclear how the underlying molecular mechanisms observed in EMT—and therefore in tumor budding—could either influence the effectiveness of chemoradiotherapy or, in turn, be influenced by chemoradiotherapy [57]. This was not, however, the purpose of the present study.
The aim of this study was to assess the impact of tumor budding, analyzed in surgical specimens, on oncological outcome and its possible role as a stratification parameter for a modified neoadjuvant therapy in chemoradiation-insensitive non-metastasized locally advanced mid-to-low rectal cancer patients.
Our data demonstrate a significant correlation between tumor budding, tumor regression, and downstaging after nCRT. In this respect, the current findings are consistent with the existing scarce literature investigating the role of tumor budding in neoadjuvantly treated rectal cancer patients (Table 6; [24, 37,38,39,40]).
In our cohort, we observed a T-level downstaging in 51.6% (65 of 126) of patients, together with an N‑level downstaging in 65.2% (43 of 66 clinically involved lymph nodes) of patients, accounting for an overall response rate (T-level or N‑level downstaging) of 64.3% (81 of 126). This is in accordance with the literature, where downstaging rates of 28–62% are reported [58, 59]. Patients with BD-0 experienced a T-level response rate of 82.6% (38 of 46) compared to those with BD-1 of 33.8% (27 of 80; P < 0.001).
Bhangu et al., who investigated the association between EMT (which is thought to be the underlying molecular mechanism behind tumor budding) and non-response in a cohort of 69 (primary: n = 54 and recurrence: n = 15) rectal cancer patients who were curatively treated with nCRT and surgery, reported a non-response rate of 65%. In the subgroup analysis of 54 primary cancers, all EMT biomarkers and tumor budding have been investigated in 45 patients and found to be positive in 24 of 31 non-responders (77.4%) compared to 1 of 14 responders (7.1%; P < 0.001), indicating a significant association with non-response [24]. Huebner et al., who investigated the impact of pathologic parameters in a cohort of 237 nCRT-treated rectal cancer patients, were the first to systematically study tumor budding in rectal cancer after neoadjuvant therapy. They found tumor budding to be a significant predictor of survival [37]. In contrast to our findings of a budding rate of 63%, they reported a budding rate of only 10%. This discrepancy may be explained by their definition of tumor budding, which was positive if any field (counted at 200 × magnification using a routine H&E staining) had 10 or more buds, a method described by Ueno et al. [60]. In our cohort, tumor budding was defined as positive (moderate or severe budding, BD-1) if one or more foci in an average of 10 HPF were observed [46, 47, 61, 62]. This method of defining tumor budding can be contrasted to that described by Sannier et al., who only recorded the absence or presence of tumor buds without any cut-off value to define tumor budding. They explained this by the ease of the applicability and the association of even low numbers of tumor buds with adverse effects on outcome in nCRT-treated patients [38]. Irrespective of the varying tumor budding rates, the abovementioned three studies—to the authors’ best knowledge—are the only existing studies that explicitly investigated the prognostic role of tumor budding in nCRT-treated rectal cancer patients (Table 6; [24, 37, 38]). Jessberger et al. demonstrated the prognostic value of tumor cell growth patterns (which included tumor budding) in predicting survival in post-CRT surgical specimens, but not in pretreatment biopsies [40]. Although this study group used a different technique to identify tumor budding, the alteration of cancer cells’ growth pattern along the invasion front by nCRT was demonstrated, indicating potential constraints in assessing posttreatment tumor budding [40].
Tumor budding is usually assessed at the tumor invasive front, as buds are most prominent here [33]. In contrast to colorectal cancer specimens without nCRT, pathological evaluation of rectal cancer after chemoradiation is more complex. It is important to consider that residual cancer cells in nCRT-treated rectal specimens are unequally distributed in the bowel wall [63], and an invasive front is not necessarily present to correctly score tumor budding in rectal cancer after nCRT [38]. In our cohort, specimens with clear radiogenic regression (AJCC/CAP grade 0, “complete response”) exhibited destruction of the tumor glandular component with subsequent fibrosis resulting in disruption of the tumor tissue, which made budding assessment more challenging, a topic first investigated and described by Du et al. [39]. Nevertheless, in the remaining regression grades (AJCC/CAP 1, 2, and 3; “moderate,” “mild,” and “poor” responses, respectively), the assessment of tumor budding was possible as the tumor invasive front could be clearly identified according to the 10 HPF method [46, 47, 62]. Du et al. in their study evaluated the morphology and prognostic value of tumor budding in 96 rectal cancer patients after radiotherapy alone and consecutive curative resection. Tumor budding in irradiated specimens was found to be an independent factor, among others, affecting long-term disease-free survival. They further demonstrated an excellent concordance of tumor budding assessed on H&E and immunohistochemical stained slides of irradiated rectal cancer specimens, indicating the feasibility of tumor budding assessment on H&E stained slides of irradiated specimens [39].
A drawback of tumor budding assessment and reporting is the limited reproducibility and the lack of standardization with various existing techniques for assessing and classifying tumor budding, as excellently reviewed recently [64,65,66]. Sources of variability in the assessment of tumor budding include the optimal location for assessment (tumor front vs. within the tumor), visualization, and staining (H&E staining vs. immunohistochemistry) of the budding cells, as well as the method of scoring (qualitative vs. quantitative) [67].
Intratumoral budding was demonstrated to be an independent prognostic factor and strongly correlated with peritumoral budding in a cohort of 511 colorectal cancer patients, supporting the future relevance of intratumoral budding, especially in preoperative rectal cancer specimens [68]. This was confirmed by a retrospective study conducted by Rogers et al., who assessed the intratumoral budding in pretreatment rectal cancer biopsies with a budding rate of 20% (18 of 89) and confirmed tumor budding to be a predictive factor for a poor pathological response to nCRT (higher ypT stage, lymph node involvement, lymphovascular invasion, and residual poorly differentiated tumors) and long-term outcome [29].
Although the evaluation of tumor budding with immunohistochemistry has improved detection rates and interobserver agreements compared to H&E staining, the International Tumor Budding Consensus Conference recommends tumor budding assessment using H&E [45]. Indeed, in some previous meta-analyses, the prognostic impact of tumor budding assessed using H&E did not differ significantly compared to immunohistochemistry [28, 33, 46, 64, 66].
Despite the existence of the aforementioned variability in the definition of how many cells constitute a tumor bud and how many buds constitute positive budding, together with the considerable interobserver variability in its reporting, most studies definitively demonstrate tumor budding as a strong negative prognostic marker in colorectal cancer [64]. Additionally, the results of the International Tumor Budding Consensus Conference (April 27–29, 2016, Bern/Switzerland) provide optimism that an agreement on an international, evidence-based standardized scoring system for tumor budding in colorectal cancer is on the horizon [45].
There are several limitations to our study. First and foremost are the limitations inherent to retrospective analyses. The number of events in relapse-free survival may be a limitation in the interpretation of the multivariate analysis. In our cohort study, the completeness of follow-up in the two groups (BD-0 and BD-1) was 88% and 81%, indicating data incompleteness and a possible bias in the analysis.
To date, the assumption that a tumor budding status following nCRT reflects its dimension before any treatment is based on one study only and should be validated in larger cohorts at other institutions [29]. Nonetheless, in the row of the few reports dealing with the role of tumor budding in neoadjuvantly treated rectal cancer patients, the present analysis provides robust data with the longest follow-up time (median: 7 years, with each patient followed at least for 5 years) and the most current treatment era (until the end of 2012) and thus treatment regimen.
Conclusion
Our data confirm the predictive value of tumor budding persistence after neoadjuvant chemoradiotherapy and, hence, putatively for the response towards nCRT in locally advanced rectal cancer patients. In addition to the established predictive parameters (TNM classification and tumor regression grading), tumor budding is accessible in pretreatment biopsy specimens and could therefore, at least hypothetically, serve as a predictor of non-response to nCRT, also providing a possibility to stratify putative non-responders into an individualized nCRT regimen.
Further efforts are needed to gather scientific evidence to validate the promising role of tumor budding and to gain a greater understanding of the underlying molecular mechanisms behind EMT and tumor budding. This information will help to improve the multidisciplinary treatment of locally advanced rectal cancer patients.
References
Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ, Dutch Colorectal Cancer Group (2001) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 345:638–646. https://doi.org/10.1056/NEJMoa010580
Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German Rectal Cancer Study Group (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351:1731–1740. https://doi.org/10.1056/NEJMoa040694
Ma B, Gao P, Wang H, Xu Q, Song Y, Huang X, Sun J, Zhao J, Luo J, Sun Y, Wang Z (2017) What has preoperative radio(chemo)therapy brought to localized rectal cancer patients in terms of perioperative and long-term outcomes over the past decades? A systematic review and meta-analysis based on 41,121 patients. Int J Cancer 141:1052–1065. https://doi.org/10.1002/ijc.30805
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC, EORTC Radiotherapy Group Trial 22921 (2006) Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 355:1114–1123. https://doi.org/10.1056/NEJMoa060829
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C (2012) Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 30:1926–1933. https://doi.org/10.1200/JCO.2011.40.1836
Fokas E, Liersch T, Fietkau R, Hohenberger W, Beissbarth T, Hess C, Becker H, Ghadimi M, Mrak K, Merkel S, Raab HR, Sauer R, Wittekind C, Rödel C (2014) Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol 32:1554–1562. https://doi.org/10.1200/JCO.2013.54.3769
Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U (2005) Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 23:5644–5650. https://doi.org/10.1200/JCO.2005.08.144
van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer Group (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12:575–582. https://doi.org/10.1016/S1470-2045(11)70097-3
Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J, Seitz JF, Buecher B, Mackiewicz R, Ducreux M, Bedenne L (2006) Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 24:4620–4625. https://doi.org/10.1200/JCO.2006.06.7629
Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavère P, Glanzmann C, Cellier P, Collette L, EORTC Radiation Oncology Group (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer. Long-term results of the EORTC 22921 randomised study. Lancet Oncol 15:184–190. https://doi.org/10.1016/S1470-2045(13)70599-0
Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B, McLachlan SA, McKendrick J, Leong T, Hartopeanu C, Zalcberg J, Mackay J (2012) Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 30:3827–3833. https://doi.org/10.1200/JCO.2012.42.9597
Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A (2017) Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 18:336–346. https://doi.org/10.1016/S1470-2045(17)30086-4
Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, Chen D, Cao J, Wei H, Peng X, Huang Z, Cai G, Zhao R, Huang Z, Xu L, Zhou H, Wei Y, Zhang H, Zheng J, Huang Y, Zhou Z, Cai Y, Kang L, Huang M, Peng J, Ren D, Wang J (2016) Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the Chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol 34:3300–3307. https://doi.org/10.1200/JCO.2016.66.6198
Fokas E, Rödel C (2017) Optimales Fraktionierungsschema und Zeitintervall zwischen Radiotherapie und Operation beim Rektumkarzinom: Die multizentrische, randomisierte Stockholm-III-Studie. Strahlenther Onkol 193:761–762. https://doi.org/10.1007/s00066-017-1181-2
Rödel C, Fokas E (2017) Inklusion von Oxaliplatin in die multimodale Behandlung des lokal fortgeschrittenen Rektumkarzinoms. Strahlenther Onkol 193:168–170. https://doi.org/10.1007/s00066-016-1090-9
Yang YJ, Cao L, Li ZW, Zhao L, Wu HF, Yue D, Yang JL, Zhou ZR, Liu SX (2016) Fluorouracil-based neoadjuvant chemoradiotherapy with or without oxaliplatin for treatment of locally advanced rectal cancer: an updated systematic review and meta-analysis. Oncotarget 7:45513–45524. https://doi.org/10.18632/oncotarget.9995
Gani C, Zips D (2017) Zeitintervall zwischen neoadjuvanter Radiochemotherapie und Operation beim Rektumkarzinom: Kein Einfluss auf die pathologische Komplettremissionsrate? Strahlenther Onkol 193:91–92. https://doi.org/10.1007/s00066-016-1080-y
Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, German Rectal Cancer Study Group (2012) Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 13:679–687. https://doi.org/10.1016/S1470-2045(12)70187-0
Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, Hofheinz RD, Ghadimi M, Wolff HA, Lang-Welzenbach M, Raab HR, Wittekind C, Ströbel P, Staib L, Wilhelm M, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R, Liersch T, German Rectal Cancer Study Group (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16:979–989. https://doi.org/10.1016/S1470-2045(15)00159-X
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E (2016) Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 34:3773–3780. https://doi.org/10.1200/JCO.2016.67.6049
Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, Michalski W, Olędzki J, Kuśnierz J, Zając L, Bednarczyk M, Szczepkowski M, Tarnowski W, Kosakowska E, Zwoliński J, Winiarek M, Wiśniowska K, Partycki M, Bęczkowska K, Polkowski W, Styliński R, Wierzbicki R, Bury P, Jankiewicz M, Paprota K, Lewicka M, Ciseł B, Skórzewska M, Mielko J, Bębenek M, Maciejczyk A, Kapturkiewicz B, Dybko A, Hajac Ł, Wojnar A, Leśniak T, Zygulska J, Jantner D, Chudyba E, Zegarski W, Las-Jankowska M, Jankowski M, Kołodziejski L, Radkowski A, Żelazowska-Omiotek U, Czeremszyńska B, Kępka L, Kolb-Sielecki J, Toczko Z, Fedorowicz Z, Dziki A, Danek A, Nawrocki G, Sopyło R, Markiewicz W, Kędzierawski P, Wydmański J, Polish Colorectal Study Group (2016) Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 27:834–842. https://doi.org/10.1093/annonc/mdw062
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL (2010) Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 11:835–844. https://doi.org/10.1016/S1470-2045(10)70172-8
Ferrari L, Fichera A (2015) Neoadjuvant chemoradiation therapy and pathological complete response in rectal cancer. Gastroenterol Rep (oxf) 3:277–288. https://doi.org/10.1093/gastro/gov039
Bhangu A, Wood G, Brown G, Darzi A, Tekkis P, Goldin R (2014) The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Dis 16:133–143. https://doi.org/10.1111/codi.12482
Edge S, Fritz A, Byrd D, Greene F, Compton C, Trotti A (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Jäger T, Neureiter D, Urbas R, Klieser E, Hitzl W, Emmanuel K, Dinnewitzer A (2017) Applicability of American Joint Committee on Cancer and College of American pathologists regression grading system in rectal cancer. Dis Colon Rectum 60:815–826. https://doi.org/10.1097/DCR.0000000000000806
Fokas E, Ströbel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG, Hartmann A, Kaufmann M, Sauer R, Graeven U, Hoffmanns H, Raab HR, Hothorn T, Wittekind C, Rödel C, German Rectal Cancer Study Group (2017) Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst 109(12).https://doi.org/10.1093/jnci/djx095
De Smedt L, Palmans S, Sagaert X (2016) Tumour budding in colorectal cancer: what do we know and what can we do? Virchows Arch 468:397–408. https://doi.org/10.1007/s00428-015-1886-5
Rogers AC, Gibbons D, Hanly AM, Hyland JM, O’Connell PR, Winter DC, Sheahan K (2014) Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 27:156–162. https://doi.org/10.1038/modpathol.2013.124
Prall F, Ostwald C (2007) Tumour budding in colorectal carcinoma. Histopathology 50:151–162. https://doi.org/10.1111/j.1365-2559.2006.02551.x
Bhangu A, Wood G, Mirnezami A, Darzi A, Tekkis P, Goldin R (2012) Epithelial mesenchymal transition in colorectal cancer: Seminal role in promoting disease progression and resistance to neoadjuvant therapy. Surg Oncol 21:316–323. https://doi.org/10.1016/j.suronc.2012.08.003
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R (2012) Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol 25:1315–1325. https://doi.org/10.1038/modpathol.2012.94
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119:1438–1449. https://doi.org/10.1172/JCI38019
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133:704–715. https://doi.org/10.1016/j.cell.2008.03.027
Morel AP, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3:e2888. https://doi.org/10.1371/journal.pone.0002888
Huebner M, Wolff BG, Smyrk TC, Aakre J, Larson DW (2012) Partial pathologic response and nodal status as most significant prognostic factors for advanced rectal cancer treated with preoperative chemoradiotherapy. World J Surg 36:675–683. https://doi.org/10.1007/s00268-011-1409-8
Sannier A, Lefèvre JH, Panis Y, Cazals-Hatem D, Bedossa P, Guedj N (2014) Pathological prognostic factors in locally advanced rectal carcinoma after neoadjuvant radiochemotherapy: analysis of 113 cases. Histopathology 65:623–630. https://doi.org/10.1111/his.12432
Du C, Xue W, Li J, Cai Y, Gu J (2012) Morphology and prognostic value of tumor budding in rectal cancer after neoadjuvant radiotherapy. Hum Pathol 43:1061–1067. https://doi.org/10.1016/j.humpath.2011.07.026
Jessberger J, Erlenbach-Wünsch K, Posselt R, Haderlein M, Agaimy A, Fietkau R, Hartmann A, Distel L (2017) Combination of growth pattern and tumor regression identifies a high-risk group in neoadjuvant treated rectal cancer patients. J Dig Dis 18:283–291. https://doi.org/10.1111/1751-2980.12471
Ofner D, Devries AF, Schaberl-Moser R, Greil R, Rabl H, Tschmelitsch J, Zitt M, Kapp KS, Fastner G, Keil F, Eisterer W, Jäger R, Offner F, Gnant M, Thaler J, TAKO 05/ABCSG R‑02 Trial Investigators (2011) Preoperative oxaliplatin, capecitabine, and external beam radiotherapy in patients with newly diagnosed, primary operable, cT3NxM0, low rectal cancer: a phase II study. Strahlenther Onkol 187:100–107. https://doi.org/10.1007/s00066-010-2182-6
Kogler P, DeVries AF, Eisterer W, Thaler J, Sölkner L, Öfner D, TAKO 05/ABCSG R‑02 Trial Investigators (2018) Intensified preoperative chemoradiation by adding oxaliplatin in locally advanced, primary operable (cT3NxM0) rectal cancer: Impact on long-term outcome. Results of the phase II TAKO 05/ABCSG R‑02 trial. Strahlenther Onkol 194:41–49. https://doi.org/10.1007/s00066-017-1219-5
Jäger T, Nawara C, Neureiter D, Holzinger J, Öfner-Velano D, Dinnewitzer A (2015) Impact of anastomotic leakage on long-term survival in mid-to-low rectal cancer. Chirurg 86:1072–1082. https://doi.org/10.1007/s00104-015-0090-0
Austrian Society of Pathology Qualitätsstandards in der Pathologie. http://www.pathology.at/images/stories/PDF/Qualitaetsstandards/QS_Kolon_Rektum_V1.0.pdf. Accessed 11 Mar 2017
Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30:1299–1311. https://doi.org/10.1038/modpathol.2017.46
Koelzer VH, Zlobec I, Lugli A (2016) Tumor budding in colorectal cancer—ready for diagnostic practice? Hum Pathol 47:4–19. https://doi.org/10.1016/j.humpath.2015.08.007
Horcic M, Koelzer VH, Karamitopoulou E, Terracciano L, Puppa G, Zlobec I, Lugli A (2013) Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol 44:697–705. https://doi.org/10.1016/j.humpath.2012.07.026
Dinnewitzer A, Jäger T, Nawara C, Buchner S, Wolfgang H, Öfner D (2013) Cumulative incidence of permanent stoma after sphincter preserving low anterior resection of mid and low rectal cancer. Dis Colon Rectum 56:1134–1142. https://doi.org/10.1097/DCR.0b013e31829ef472
Clark TG, Altman DG, De Stavola BL (2002) Quantification of the completeness of follow-up. Lancet 359:1309–1310. https://doi.org/10.1016/S0140-6736(02)08272-7
Mace AG, Pai RK, Stocchi L, Kalady MF (2015) American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum 58:32–44. https://doi.org/10.1097/DCR.0000000000000266
Cao H, Xu E, Liu H, Wan L, Lai M (2015) Epithelial-mesenchymal transition in colorectal cancer metastasis: a system review. Pathol Res Pract 211:557–569. https://doi.org/10.1016/j.prp.2015.05.010
Kirchner T, Brabletz T (2000) Patterning and nuclear β‑Catenin expression in the colonic adenoma-carcinoma sequence: analogies with embryonic gastrulation. Am J Pathol 157:1113–1121. https://doi.org/10.1016/S0002-9440(10)64626-3
Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1:651–661. https://doi.org/10.18632/oncotarget.199
Wang L, Zhang XM, Li Z, Liu XJ, Chai J, Zhang GY, Cheng YF (2013) Overexpression of nuclear β‑catenin in rectal adenocarcinoma is associated with radioresistance. World J Gastroenterol 19:6876–6882. https://doi.org/10.3748/wjg.v19.i40.6876
Takahashi H, Nakamura K, Usami A, Tsuruta T, Hashimura M, Matsumoto T, Saegusa M (2017) Possible role of nuclear β‑catenin in resistance to preoperative chemoradiotherapy in locally advanced rectal cancer. Histopathology 71:227–237. https://doi.org/10.1111/his.13227
Kawamoto A, Yokoe T, Tanaka K, Saigusa S, Toiyama Y, Yasuda H, Inoue Y, Miki C, Kusunoki M (2012) Radiation induces epithelial-mesenchymal transition in colorectal cancer cells. Oncol Rep 27:51–57. https://doi.org/10.3892/or.2011.1485
Tato-Costa J, Casimiro S, Pacheco T, Pires R, Fernandes A, Alho I, Pereira P, Costa P, Castelo HB, Ferreira J, Costa L (2016) Therapy-induced cellular senescence induces epithelial-to-mesenchymal transition and increases invasiveness in rectal cancer. Clin Colorectal Cancer 15:170–178.e3. https://doi.org/10.1016/j.clcc.2015.09.003
Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, Rich TA, Skibber J (1999) Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 44:1027–1038. https://doi.org/10.1016/S0360-3016(99)00099-1
Medich D, McGinty J, Parda D, Karlovits S, Davis C, Caushaj P, Lembersky B (2001) Preoperative chemoradiotherapy and radical surgery for locally advanced distal rectal adenocarcinoma. Dis Colon Rectum 44:1123–1128. https://doi.org/10.1007/BF02234632
Ueno H, Mochizuki H, Shinto E, Hashiguchi Y, Hase K, Talbot IC (2002) Histologic indices in biopsy specimens for estimating the probability of extended local spread in patients with rectal carcinoma. Cancer 94:2882–2891. https://doi.org/10.1002/cncr.10551
Lugli A, Karamitopoulou E, Zlobec I (2012) Tumour budding: a promising parameter in colorectal cancer. Br J Cancer 106:1713–1717. https://doi.org/10.1038/bjc.2012.127
Karamitopoulou E, Zlobec I, Kölzer V, Kondi-Pafiti A, Patsouris ES, Gennatas K, Lugli A (2013) Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol 26:295–301. https://doi.org/10.1038/modpathol.2012.155
Tranchart H, Lefèvre JH, Svrcek M, Flejou JF, Tiret E, Parc Y (2013) What is the incidence of metastatic lymph node involvement after significant pathologic response of primary tumor following neoadjuvant treatment for locally advanced rectal cancer? Ann Surg Oncol 20:1551–1559. https://doi.org/10.1245/s10434-012-2773-9
Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K (2016) Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer 115:831–840. https://doi.org/10.1038/bjc.2016.274
Grigore AD, Jolly MK, Jia D, Farach-Carson MC, Levine H (2016) Tumor budding: the name is EMT. Partial EMT. J Clin Med 5(5).https://doi.org/10.3390/jcm5050051
van Wyk HC, Park J, Roxburgh C, Horgan P, Foulis A, McMillan DC (2015) The role of tumour budding in predicting survival in patients with primary operable colorectal cancer: a systematic review. Cancer Treat Rev 41:151–159. https://doi.org/10.1016/j.ctrv.2014.12.007
Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, Uchimoto K, Nakamura S, Nonomura A, Nakajima Y (2008) Prognostic value of Immunohistochemical analysis of tumor budding in colorectal carcinoma. Anticancer Res 28:1831–1836
Lugli A, Vlajnic T, Giger O, Karamitopoulou E, Patsouris ES, Peros G, Terracciano LM, Zlobec I (2011) Intratumoral budding as a potential parameter of tumor progression in mismatch repair-proficient and mismatch repair-deficient colorectal cancer patients. Hum Pathol 42:1833–1840. https://doi.org/10.1016/j.humpath.2011.02.010
Funding
Open access funding provided by Paracelsus Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T. Jäger, D. Neureiter, M. Fallaha, P. Schredl, T. Kiesslich, R. Urbas, E. Klieser, J. Holzinger, F. Sedlmayer, K. Emmanuel, and A. Dinnewitzer declare that they have no conflict of interest.
Additional information
Tarkan Jäger and Daniel Neureiter contributed equally to this work.
A preliminary evaluation of the data shown in this manuscript was presented as a scientific poster at the 47th World Congress of Surgery 2017 in Basel, Switzerland, 13–17 August 2017.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jäger, T., Neureiter, D., Fallaha, M. et al. The potential predictive value of tumor budding for neoadjuvant chemoradiotherapy response in locally advanced rectal cancer. Strahlenther Onkol 194, 991–1006 (2018). https://doi.org/10.1007/s00066-018-1340-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1340-0
Keywords
- Neoadjuvant therapy
- Tumor microenvironment
- Epithelial–mesenchymal transition
- Colorectal cancer
- Prognostic factor