Abstract
Purpose
This systematic review evaluates the completeness of dosimetric features and their inclusion as covariates in genetic-toxicity association studies.
Materials and methods
Original research studies associating genetic features and normal tissue complications following radiotherapy were identified from PubMed. The use of dosimetric data was determined by mining the statement of prescription dose, dose fractionation, target volume selection or arrangement and dose distribution. The consideration of the dosimetric data as covariates was based on the statement mentioned in the statistical analysis section. The significance of these covariates was extracted from the results section. Descriptive analyses were performed to determine their completeness and inclusion as covariates.
Results
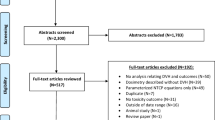
A total of 174 studies were found to satisfy the inclusion criteria. Studies published ≥2010 showed increased use of dose distribution information (p = 0.07). 33% of studies did not include any dose features in the analysis of gene-toxicity associations. Only 29% included dose distribution features as covariates and reported the results. 59% of studies which included dose distribution features found significant associations to toxicity.
Conclusion
A large proportion of studies on the correlation of genetic markers with radiotherapy-related side effects considered no dosimetric parameters. Significance of dose distribution features was found in more than half of the studies including these features, emphasizing their importance. Completeness of radiation-specific clinical data may have increased in recent years which may improve gene-toxicity association studies.
Zusammenfassung
Zielsetzung
Dieser systematische Review untersucht die Verwendung und den Nutzen dosimetrischer Parameter als Kovariaten in Studien, die genetische Informationen mit dem Auftreten von Nebenwirkungen der Strahlentherapie assoziieren.
Material und Methoden
Originalarbeiten, die genetische Parameter mit Normalgewebekomplikationen nach Strahlentherapie verknüpfen, wurden unter Verwendung von PubMed identifiziert. Die Verwendung von dosimetrischen Daten wurde anhand von Angaben zur verschriebenen Dosis, der Fraktionierung, der Zielvolumenauswahl oder der Dosisverteilung geprüft. Ob diese Daten als Kovariaten in der Modellierung zur Anwendung kamen, wurde im Statistikteil der jeweiligen Arbeit geprüft. Die Korrelation mit der betrachteten Nebenwirkung wurde dem Ergebnissteil entnommen. Mittels deskriptiver Statistik wurden die Verwendung von dosimetrischen Parametern und deren prognostischer Wert zusammengefasst.
Ergebnisse
Insgesamt 174 Studien erfüllten die Einschlusskriterien. Studien, die ab 2010 veröffentlicht wurden, schlossen häufiger Informationen zur Dosisverteilung ein (p = 0,07). Von den Studien zur Vorhersage von Nebenwirkungen anhand von genetischen Daten berücksichtigten 33 % keine Dosisparameter. Nur 29 % schlossen dosimetrische Parameter ein und zeigten deren Ergebnisse. In 59 % dieser Studien waren die dosimetrischen Parameter signifikant mit der betrachteten Nebenwirkung korreliert.
Schlussfolgerung
Ein großer Anteil an Studien zur Korrelation von genetischen Markern mit strahlentherapiebedingten Nebenwirkungen berücksichtigte keine dosimetrischen Parameter. Ein signifikanter Einfluss dieser Parameter wurde jedoch in über 50 % aller Studien gefunden, die diese eingeschlossen hatten, was deren Relevanz unterstreicht. Die Nutzung dosimetrischer Parameter hat sich in den letzten Jahren erhöht, was zur Verbesserung entsprechender Studien geführt haben könnte.

Similar content being viewed by others
References
Kerns SL, Kundu S, Oh JH et al (2015) The prediction of radiotherapy toxicity using single nucleotide polymorphism—based models: a step toward prevention. Semin Radiat Oncol. https://doi.org/10.1016/j.semradonc.2015.05.006
Rosenstein BS (2017) Radiogenomics: identification of genomic predictors for radiation toxicity. Semin Radiat Oncol 27:300–309
Rosenstein BS, West CM, Bentzen SM et al (2014) Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys 89:709–713
Reuther S, Szymczak S, Raabe A et al (2014) Association between SNPs in defined functional pathways and risk of early or late toxicity as well as individual radiosensitivity. Strahlenther Onkol 191:59–66
Lambin P, van Stiphout RGPM, Starmans MHW et al (2012) Predicting outcomes in radiation oncology—multifactorial decision support systems. Nat Rev Clin Oncol 10:27–40
Peeken JC, Nüsslin F, Combs SE (2017) “Radio-oncomics”. Strahlenther Onkol 193:767–779
Matsuo Y, Shibuya K, Nakamura M et al (2012) Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 83:e545–e549
Wang S, Liao Z, Wei X et al (2006) Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 66:1399–1407
Asakura H, Hashimoto T, Zenda S et al (2010) Analysis of dose–volume histogram parameters for radiation pneumonitis after definitive concurrent chemoradiotherapy for esophageal cancer. Radiother Oncol 95:240–244
Yahya N, Ebert MA, Bulsara M et al (2015) Urinary symptoms following external beam radiotherapy of the prostate: dose–symptom correlates with multiple-event and event-count models. Radiother Oncol 117:277–282
Valdagni R, Vavassori V, Rancati T et al (2012) Increasing the risk of late rectal bleeding after high-dose radiotherapy for prostate cancer: the case of previous abdominal surgery. Results from a prospective trial. Radiother Oncol 103:252–255
Roeder F, Friedrich J, Timke C et al (2010) Correlation of patient-related factors and dose-volume histogram parameters with the onset of radiation pneumonitis in patients with small cell lung cancer. Strahlenther Onkol 186:149–156
Yoon H, Oh D, Park HC et al (2013) Predictive factors for gastroduodenal toxicity based on endoscopy following radiotherapy in patients with hepatocellular carcinoma. Strahlenther Onkol 189:541–546
Takahashi S, Go T, Kasai Y, Yokomise H, Shibata T (2016) Relationship between dose–volume parameters and pulmonary complications after neoadjuvant chemoradiotherapy followed by surgery for lung cancer. Strahlenther Onkol 192:658–667
Chen WS, Townsend JP, James BY (2017) Radiation-specific clinical data should be included in existing large-scale genomic datasets. Int J Radiat Oncol Biol Phys 98:8–10
Guo Z, Shu Y, Wang H, Zhou H, Zhang W (2015) Radiogenomics helps to achieve personalized therapy by evaluating patient responses to radiation treatment. Carcinogenesis 36:307–317
Bentzen SM, Parliament M, Deasy JO et al (2010) Biomarkers and surrogate endpoints for normal-tissue effects of radiation therapy: the importance of dose–volume effects. Int J Radiat Oncol Biol Phys 76:S145–S150
Dörr W, Herrmann T, Baumann M (2014) Application of organ tolerance dose-constraints in clinical studies in radiation oncology. Strahlenther Onkol 190:621–627
West CM, Barnett GC (2011) Genetics and genomics of radiotherapy toxicity: towards prediction. Genome Med 3:1
Kerns SL, De Ruysscher D, Andreassen CN et al (2014) STROGAR—strengthening the reporting of genetic association studies in radiogenomics. Radiother Oncol 110:182–188
Bentzen SM, Constine LS, Deasy JO et al (2010) Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 76:S3–S9
Mehta V (2005) Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 63:5–24
Roach M 3rd, Gandara DR, Yuo HS et al (1995) Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol 13:2606–2612
Zhao J, Yorke ED, Li L et al (2016) Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: a pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys 95:1357–1366
Marks LB, Bentzen SM, Deasy JO et al (2010) Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys 76:S70–S76
Yahya N, Ebert MA, House MJ et al (2017) Modeling urinary dysfunction after external beam radiation therapy of the prostate using bladder dose-surface maps: evidence of spatially variable response of the bladder surface. Int J Radiat Oncol Biol Phys 97:420–426
Wortel RC, Witte MG, van der Heide UA et al (2015) Dose–surface maps identifying local dose-effects for acute gastrointestinal toxicity after radiotherapy for prostate cancer. Radiother Oncol 117:515–520
Acknowledgments
We acknowledge funding from the National University of Malaysia (GGPM-2017-095). We thank Steffen Lock and Muaz Azhari for the German translations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Yahya, X.J. Chua, H.A. Manan and F. Ismail declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Caption Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Yahya, N., Chua, XJ., Manan, H.A. et al. Inclusion of dosimetric data as covariates in toxicity-related radiogenomic studies. Strahlenther Onkol 194, 780–786 (2018). https://doi.org/10.1007/s00066-018-1303-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-018-1303-5
Keywords
- Radiotherapy
- Genetic association studies
- Dose-response relationship, radiation
- Radiation effects
- Genetic testing