Abstract
Purpose
Surgical treatment of head and neck malignancies frequently includes microvascular free tissue transfer. Preoperative radiotherapy increases postoperative fibrosis-related complications up to transplant loss. Fibrogenesis is associated with re-expression of embryonic preserved tissue developmental mediators: osteopontin (OPN), regulated by sex-determining region Y‑box 9 (Sox9), and homeobox A9 (HoxA9) play important roles in pathologic tissue remodeling and are upregulated in atherosclerotic vascular lesions; dickkopf-1 (DKK1) inhibits pro-fibrotic and atherogenic Wnt signaling. We evaluated the influence of irradiation on expression of these mediators in arteries of the head and neck region.
Materials and methods
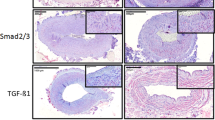
DKK1, HoxA9, OPN, and Sox9 expression was examined immunohistochemically in 24 irradiated and 24 nonirradiated arteries of the lower head and neck region. The ratio of positive cells to total cell number (labeling index) in the investigated vessel walls was assessed semiquantitatively.
Results
DKK1 expression was significantly decreased, whereas HoxA9, OPN, and Sox9 expression were significantly increased in irradiated compared to nonirradiated arterial vessels.
Conclusion
Preoperative radiotherapy induces re-expression of embryonic preserved mediators in arterial vessels and may thus contribute to enhanced activation of pro-fibrotic downstream signaling leading to media hypertrophy and intima degeneration comparable to fibrotic development steps in atherosclerosis. These histopathological changes may be promoted by HoxA9-, OPN-, and Sox9-related inflammation and vascular remodeling, supported by downregulation of anti-fibrotic DKK1. Future pharmaceutical strategies targeting these vessel alterations, e. g., bisphosphonates, might reduce postoperative complications in free tissue transfer.
Zusammenfassung
Zielsetzung
Die operative Behandlung von Tumoren im Kopf- und Halsbereich umfasst den Transfer mikrovaskulärer Gewebetransplantate. Präoperative Bestrahlung verursacht eine erhöhte Inzidenz fibrosebedingter Komplikationen bis zum Transplantatverlust. Die Fibroseentstehung ist mit der Reexpression embryonal konservierter, in der Gewebeentwicklung relevanter Mediatoren assoziiert: Osteopontin (OPN), reguliert von Sex determining region Y‑box 9 (Sox9), und Homeobox A9 (HoxA9) spielen wichtige Rollen im pathologischen Gewebeumbau und sind hochreguliert in arteriosklerotischen Gefäßläsionen; Dickkopf-1 (DKK1) inhibiert den profibrotischen, atherogenen Wnt-Signalweg. Wir untersuchten den Einfluss der Bestrahlung auf die Expression dieser Mediatoren in Arterien der Kopf- und Halsregion.
Material und Methoden
Die Expression von DKK1, HoxA9, OPN und Sox9 wurde in 24 bestrahlten und 24 nichtbestrahlten Arterien der unteren Kopf- und Halsregion immunhistochemisch untersucht. Das Verhältnis positiver Zellen zur Gesamtzellzahl (Färbungsindex) in den untersuchten Gefäßwänden wurde semiquantitativ bestimmt.
Ergebnisse
Die Expression von DKK1 war in bestrahlten im Vergleich zu nichtbestrahlten arteriellen Gefäßen signifikant erniedrigt, die von HoxA9, OPN und Sox9 hingegen signifikant erhöht.
Schlussfolgerung
Präoperative Bestrahlung induziert die Reexpression embryonal konservierter Mediatoren in arteriellen Gefäßen und könnte somit zur verstärkten Aktivierung des profibrotischen Downstreams beitragen, was zu Mediahypertrophie und Intimadegeneration, vergleichbar mit fibrotischen Entwicklungsstufen bei Arteriosklerose, führt. Diese histopathologischen Veränderungen könnten durch HoxA9-, OPN- und Sox9-beeinflusste Inflammation und Gefäßumbau begünstigt werden, unterstützt durch die Herabregulierung des antifibrotischen DKK1. Pharmazeutische Strategien gegen derartige Gefäßveränderungen, z. B. mit Bisphosphonaten, könnten postoperative Komplikationen nach freiem Gewebetransfer reduzieren.



Similar content being viewed by others
References
Preidl RH, Wehrhan F, Schlittenbauer T, Neukam FW, Stockmann P (2015) Perioperative factors that influence the outcome of microsurgical reconstructions in craniomaxillofacial surgery. Br J Oral Maxillofac Surg. doi:10.1016/j.bjoms.2015.03.007
Schultze-Mosgau S, Wehrhan F, Amann K, Radespiel-Troger M, Rodel F, Grabenbauer GG (2003) In Vivo TGF-beta 3 expression during wound healing in irradiated tissue. An experimental study. Strahlenther Onkol 179(6):410–416. doi:10.1007/s00066-003-1049-5
Schultze-Mosgau S, Rodel F, Radespiel-Troger M, Worl J, Grabenbauer GG, Neukam FW (2002) Vascularization of the area between free grafts and irradiated graft beds in the neck in rats. Br J Oral Maxillofac Surg 40(1):37–44. doi:10.1054/bjom.2001.0651
Schultze-Mosgau S, Wehrhan F, Rodel F, Amann K, Radespiel-Troger M, Grabenbauer GG (2003) Transforming growth factor-beta receptor-II up-regulation during wound healing in previously irradiated graft beds in vivo. Wound Repair Regen 11(4):297–305
Kasper M, Fehrenbach H (2000) Immunohistochemical evidence for the occurrence of similar epithelial phenotypes during lung development and radiation-induced fibrogenesis. Int J Radiat Biol 76(4):493–501
Kalluri R, Neilson EG (2003) Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112(12):1776–1784. doi:10.1172/JCI20530
Sas-Korczynska B, Luczynska E, Kamzol W, Sokolowski A (2016) Analysis of risk factors for pulmonary complications in patients with limited-stage small cell lung cancer: a single-centre retrospective study. Strahlenther Onkol. doi:10.1007/s00066-016-1069-6
Sun Z, Wang C, Shi C, Sun F, Xu X, Qian W, Nie S, Han X (2014) Activated Wnt signaling induces myofibroblast differentiation of mesenchymal stem cells, contributing to pulmonary fibrosis. Int J Mol Med 33(5):1097–1109. doi:10.3892/ijmm.2014.1672
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391(6665):357–362. doi:10.1038/34848
Trigueros-Motos L, Gonzalez-Granado JM, Cheung C, Fernandez P, Sanchez-Cabo F, Dopazo A, Sinha S, Andres V (2013) Embryological-origin-dependent differences in homeobox expression in adult aorta: role in regional phenotypic variability and regulation of NF-kappaB activity. Arterioscler Thromb Vasc Biol 33(6):1248–1256. doi:10.1161/ATVBAHA.112.300539
Kawai T, Yasuchika K, Ishii T, Miyauchi Y, Kojima H, Yamaoka R, Katayama H, Yoshitoshi EY, Ogiso S, Kita S, Yasuda K, Fukumitsu K, Komori J, Hatano E, Kawaguchi Y, Uemoto S (2016) SOX9 is a novel cancer stem cell marker surrogated by osteopontin in human hepatocellular carcinoma. Sci Rep 6:30489. doi:10.1038/srep30489
Pritchett J, Harvey E, Athwal V, Berry A, Rowe C, Oakley F, Moles A, Mann DA, Bobola N, Sharrocks AD, Thomson BJ, Zaitoun AM, Irving WL, Guha IN, Hanley NA, Hanley KP (2012) Osteopontin is a novel downstream target of SOX9 with diagnostic implications for progression of liver fibrosis in humans. Hepatology 56(3):1108–1116. doi:10.1002/hep.25758
Kilic G, Wang J, Sosa-Pineda B (2006) Osteopontin is a novel marker of pancreatic ductal tissues and of undifferentiated pancreatic precursors in mice. Dev Dyn 235(6):1659–1667. doi:10.1002/dvdy.20729
Piera-Velazquez S, Mendoza FA, Jimenez SA (2016) Endothelial to Mesenchymal Transition (EndoMT) in the Pathogenesis of Human Fibrotic Diseases. J Clin Med 5(4). doi:10.3390/jcm5040045
Ferrell CM, Dorsam ST, Ohta H, Humphries RK, Derynck MK, Haqq C, Largman C, Lawrence HJ (2005) Activation of stem-cell specific genes by HOXA9 and HOXA10 homeodomain proteins in CD34+ human cord blood cells. Stem Cells 23(5):644–655. doi:10.1634/stemcells.2004-0198
Bastakoty D, Young PP (2016) Wnt/beta-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J 30(10):3271–3284. doi:10.1096/fj.201600502R
Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S (2015) The dark and bright side of atherosclerotic calcification. Atherosclerosis 238(2):220–230. doi:10.1016/j.atherosclerosis.2014.12.011
Scheraga RG, Thannickal VJ (2014) Wnt/beta-catenin and transforming growth factor-beta signaling in pulmonary fibrosis. A case for antagonistic pleiotropy? Am J Respir Crit Care Med 190(2):129–131. doi:10.1164/rccm.201406-1037ED
Lin H, Angeli M, Chung KJ, Ejimadu C, Rivera Rosa A, Lee T (2016) sFRP2 activates Wnt/beta-catenin signaling in cardiac fibroblasts: differential roles in cell growth, energy metabolism and extracellular matrix remodeling. Am J Physiol, Cell Physiol 00137:02016. doi:10.1152/ajpcell.00137.2016
Blyszczuk P, Muller-Edenborn B, Valenta T, Osto E, Stellato M, Behnke S, Glatz K, Basler K, Luscher TF, Distler O, Eriksson U, Kania G (2016) Transforming growth factor-beta-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. Eur Heart J. doi:10.1093/eurheartj/ehw116
Straub JM, New J, Hamilton CD, Lominska C, Shnayder Y, Thomas SM (2015) Radiation-induced fibrosis: mechanisms and implications for therapy. J Cancer Res Clin Oncol 141(11):1985–1994. doi:10.1007/s00432-015-1974-6
Scott AS, Parr LA, Johnstone PA (2009) Risk of cerebrovascular events after neck and supraclavicular radiotherapy: a systematic review. Radiother Oncol 90(2):163–165. doi:10.1016/j.radonc.2008.12.019
Youn SW, Park KK (2015) Small-nucleic-acid-based therapeutic strategy targeting the transcription factors regulating the vascular inflammation, remodeling and fibrosis in atherosclerosis. Int J Mol Sci 16(5):11804–11833. doi:10.3390/ijms160511804
Cantile M, Schiavo G, Terracciano L, Cillo C (2008) Homeobox genes in normal and abnormal vasculogenesis. Nutr Metab Cardiovasc Dis 18(10):651–658. doi:10.1016/j.numecd.2008.08.001
Patties I, Haagen J, Dorr W, Hildebrandt G, Glasow A (2015) Late inflammatory and thrombotic changes in irradiated hearts of C57BL/6 wild-type and atherosclerosis-prone ApoE-deficient mice. Strahlenther Onkol 191(2):172–179. doi:10.1007/s00066-014-0745-7
Halle M, Gabrielsen A, Paulsson-Berne G, Gahm C, Agardh HE, Farnebo F, Tornvall P (2010) Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol 55(12):1227–1236. doi:10.1016/j.jacc.2009.10.047
Alvarado-Ruiz L, Martinez-Silva MG, Torres-Reyes LA, Pina-Sanchez P, Ortiz-Lazareno P, Bravo-Cuellar A, Aguilar-Lemarroy A, Jave-Suarez LF (2016) HOXA9 is underexpressed in cervical cancer cells and its restoration decreases proliferation, migration and expression of epithelial-to-mesenchymal transition genes. Asian Pac J Cancer Prev 17(3):1037–1047
Kothari AN, Arffa ML, Chang V, Blackwell RH, Syn WK, Zhang J, Mi Z, Kuo PC (2016) Osteopontin – a master regulator of epithelial-mesenchymal transition. J Clin Med 5(4). doi:10.3390/jcm5040039
Giachelli CM, Liaw L, Murry CE, Schwartz SM, Almeida M (1995) Osteopontin expression in cardiovascular diseases. Ann N Y Acad Sci 760:109–126
Ding Y, Chen J, Cui G, Wei Y, Lu C, Wang L, Diao H (2016) Pathophysiological role of osteopontin and angiotensin II in atherosclerosis. Biochem Biophys Res Commun 471(1):5–9. doi:10.1016/j.bbrc.2016.01.142
Wang X, Lopategi A, Ge X, Lu Y, Kitamura N, Urtasun R, Leung TM, Fiel MI, Nieto N (2014) Osteopontin induces ductular reaction contributing to liver fibrosis. Gut 63(11):1805–1818. doi:10.1136/gutjnl-2013-306373
Mucke T, Rau A, Weitz J, Ljubic A, Rohleder N, Wolff KD, Mitchell DA, Kesting MR (2012) Influence of irradiation and oncologic surgery on head and neck microsurgical reconstructions. Oral Oncol 48(4):367–371. doi:10.1016/j.oraloncology.2011.11.013
Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T (2004) DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 23(52):8520–8526. doi:10.1038/sj.onc.1207892
Lieven O, Knobloch J, Ruther U (2010) The regulation of Dkk1 expression during embryonic development. Dev Biol 340(2):256–268. doi:10.1016/j.ydbio.2010.01.037
Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, Lin NY, Beyer C, Distler O, Schett G, Distler JH (2014) The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann Rheum Dis 73(6):1232–1239. doi:10.1136/annrheumdis-2012-203194
Preidl RH, Mobius P, Weber M, Amann K, Neukam FW, Schlegel A, Wehrhan F (2014) Expression of transforming growth factor beta 1‑related signaling proteins in irradiated vessels. Strahlenther Onkol. doi:10.1007/s00066-014-0797-8
Wehrhan F, Grabenbauer GG, Rodel F, Amann K, Schultze-Mosgau S (2004) Exogenous modulation of TGF-beta(1) influences TGF-betaR-III-associated vascularization during wound healing in irradiated tissue. Strahlenther Onkol 180(8):526–533. doi:10.1007/s00066-004-1212-7
Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener MO, MacDougald OA, Distler O, Schett G, Distler JH (2012) Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis. Nat Commun 3:735. doi:10.1038/ncomms1734
Trivedi CM, Patel RC, Patel CV (2007) Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent activation of endothelium. Atherosclerosis 195(2):e50–60. doi:10.1016/j.atherosclerosis.2007.04.055
Gorski DH, Walsh K (2000) The role of homeobox genes in vascular remodeling and angiogenesis. Circ Res 87(10):865–872
Bruderer M, Alini M, Stoddart MJ (2013) Role of HOXA9 and VEZF1 in endothelial biology. J Vasc Res 50(4):265–278. doi:10.1159/000353287
Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, Postma DS, Meurs H, Heijink IH, Gosens R, Konigshoff M (2016) TGF-beta-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. doi:10.1096/fj.201500129
Cho HJ, Cho HJ, Kim HS (2009) Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep 11(3):206–213
Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA (2003) Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest 112(9):1318–1331. doi:10.1172/JCI18141
Kang N, Ng CS, Hu J, Qiu ZB, Underwood MJ, Jeremy JY, Wan S (2012) Role of osteopontin in the development of neointimal hyperplasia in vein grafts. Eur J Cardiothorac Surg 41(6):1384–1389. doi:10.1093/ejcts/ezr200
Arriazu E, Ge X, Leung TM, Magdaleno F, Lopategi A, Lu Y, Kitamura N, Urtasun R, Theise N, Antoine DJ, Nieto N (2016) Signalling via the osteopontin and high mobility group box-1 axis drives the fibrogenic response to liver injury. Gut. doi:10.1136/gutjnl-2015-310752
Wolak T (2014) Osteopontin – a multi-modal marker and mediator in atherosclerotic vascular disease. Atherosclerosis 236(2):327–337. doi:10.1016/j.atherosclerosis.2014.07.004
Jo A, Denduluri S, Zhang B, Wang Z, Yin L, Yan Z, Kang R, Shi LL, Mok J, Lee MJ, Haydon RC (2014) The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis 1(2):149–161. doi:10.1016/j.gendis.2014.09.004
Wu L, Zhu L, Shi WH, Zhang J, Ma D, Yu B (2009) Zoledronate inhibits the proliferation, adhesion and migration of vascular smooth muscle cells. Eur J Pharmacol 602(1):124–131. doi:10.1016/j.ejphar.2008.10.043
Ylitalo R (2000) Bisphosphonates and atherosclerosis. Gen Pharmacol 35(6):287–296
Funding
The present study was financially supported by the “ELAN Fonds der Universität Erlangen”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
P. Möbius, R.H.M. Preidl, M. Weber, K. Amann, F.W. Neukam, and F. Wehrhan declare that they have no competing interests.
Ethical standards
All studies on humans presented in this manuscript were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration of 1975 (in its current, revised form). Ethical aspects of the present study are approved by the ethics committee of the University of Erlangen-Nuremberg, Germany (Ref.-No. 83_13B).
Additional information
Author Contribution
P. Möbius, R.H.M. Preidl, and F. Wehrhan designed the study. P. Möbius, R.H.M. Preidl, and M. Weber collected the data. P. Möbius wrote the manuscript. F. Wehrhan formulated the hypothesis and contributed to the manuscript. M. Weber critically reviewed the article and contributed to the discussion. K. Amann helped validating the markers and contributed to the discussion. F.W. Neukam contributed to the discussion and critically reviewed the article. All authors approved the final version.
Shared first authors: P. Möbius and R.H.M. Preidl.
The present work was performed in (partial) fulfillment of the requirements for obtaining the degree “Dr. med.” (P. Möbius).
Rights and permissions
About this article
Cite this article
Möbius, P., Preidl, R.H.M., Weber, M. et al. Re-expression of pro-fibrotic, embryonic preserved mediators in irradiated arterial vessels of the head and neck region. Strahlenther Onkol 193, 951–960 (2017). https://doi.org/10.1007/s00066-017-1192-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-017-1192-z