Abstract
Purpose
To evaluate the outcomes with respect to long-term survival and toxicity in patients with nasopharyngeal carcinoma (NPC) treated in a European country with low incidence.
Materials and methods
A prospective observational study carried out by the AIRO Head and Neck group in 12 Italian institutions included 136 consecutive patients treated with radiotherapy (RT) ± chemotherapy (CHT) for NPC (without distant metastasis) between January 1, 2008 and December 31, 2010.
Results
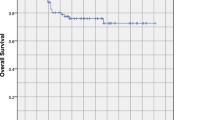
The disease-specific survival (DSS), overall survival (OS), and disease-free survival (DFS) at 5 years were 92 (±2), 91 (±3), and 69 % (±5 %), respectively. Distant failure was the most frequent modality of relapse. The local, regional, and locoregional control at 5 years were 89 (±3), 93 (±3), and 84 % (±4 %), respectively. The incidence of acute and late toxicity and the correlations with different clinical/technical variables were analyzed. Neoadjuvant CHT prolongs radiotherapy overall treatment time (OTT) and decreases treatment adherence during concomitant chemoradiotherapy. An adequate minimum dose coverage to PTV(T) is a predictive variable well related to outcome.
Conclusion
Our data do not substantially differ in terms of survival and toxicity outcomes from those reported in larger series of patients treated in countries with higher incidences of NPC. The T stage (TNM 2002 UICC classification) is predictive of DSS and OS. The GTV volume (T ± N) and an adequate minimum PTV(T) coverage dose (D95 %) were also identified as potential predictive variables. Sophisticated technologies of dose delivery (IMRT) with image-guided radiotherapy could help to obtain better minimum PTV(T) coverage dose with increased DFS; distant metastasis after treatment still remains an unresolved issue.
Zusammenfassung
Ziel
Bewertung von langfristigem Überleben und Toxizität bei Patienten mit Nasopharynxkarzinom (NPC), die in einem europäischen Land mit geringer Inzidenz behandelt wurden.
Materialien und Methoden
Die prospektive Beobachtungsanalyse, durchgeführt von der AIRO Kopf- und Hals-Gruppe an 12 italienischen Zentren, beinhaltete 136 Folgepatienten, die zwischen 1. Januar 2008 und 31. Dezember 2010 wegen NPC (ohne Fernmetastasen) mit Strahlentherapie (RT) ± Chemotherapie (CHT) behandelt wurden.
Ergebnisse
Die krankheitsspezifische Überlebensrate (DSS), das Gesamtüberleben (OS) und krankheitsfreie Überleben (DFS) nach 5 Jahren waren jeweils 92 % (±2 %), 91 % (±3 %) und 69 % (±5 %). Die häufigsten Rezidive waren Fernmetastasen. Die Kontrollwahrscheinlichkeit liegt lokal, regional und lokoregional nach 5 Jahren bei 89 % (±3 %), 93 % (±3 %) und 84 % (±4 %). Die Inzidenz der akuten und späten Toxizität und Korrelationen mit verschiedenen klinisch/technischen Variablen wurden analysiert. Neoadjuvante CHT verlängert die Zeit der Gesamtbehandlung und reduziert die Therapiecompliance während der zusammen durchgeführten Radiochemotherapie. Eine angemessene Minimaldosis von PTV(T) ist eine prädiktive Variable, die gut mit der Überlebensquote korreliert.
Schlussfolgerung
Unsere Daten zeigen keinen großen Unterschied bezüglich der Überlebensquote und Toxizität im Vergleich zu denen aus Ländern mit höherer NPC-Inzidenz. Das T‑Stadium (TNM 2002 UICC-Klassifikation) ist prädiktiv für DSS und OS. Das GTV-Volumen (T ± N) und eine ausreichende Mindestdosis an PTV(T) (D95 %) sind zusätzliche potenziell prädiktive Variablen. Weiterentwickelte IMRT-Technologien zur Dosisverabreichung mittels bildgestützter Strahlentherapie könnten zu besserer PTV(T)-Minimaldosis mit erhöhter DFS führen; Fernmetastasen nach der Behandlung bleiben ein ungelöstes Problem.

Similar content being viewed by others
References
Parkin DM, Whelan SL, Felay J, Teppo L, Tomas DB (eds) (2002) Cancer incidence in Five Continents vol. VIII. IARC Scientific Publication, Lyon, p 155
Al-Sarraf M, LeBlanc M, Giri PG et al (1998) Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 16:1310–1317
Lee AW, Tung SY, Chua DT, Ngan RK (2010) Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 102(15):1188–1198
Lee AW, Tung SY, Chan ATC et al (2011) A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Radiother Oncol 98:15–22
Hui EP, Ma BB, Leung SF et al (2009) Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neo-adjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol 27(2):242–249
Lee AW, Tung ST, Ngan RCK et al (2011) Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for loco-regional advanced nasopharyngeal carcinoma: Combined analyses of NPC-9901 and NPC-9902 trials. Eur J Cancer 47:656–666
Palazzi M, Guzzo M, Tomatis S, Cerrotta A, Potepan P, Quattrone P et al (2004) Improved outcome of nasopharyngeal carcinoma treated with conventional radiotherapy. Int J Radiat Oncol Biol Phys 60(5):1451–1458
Tonoli S, Magrini SM, Costa L, Paiar F, Simontacchi G, Scotti V, Pasinetti N, Barca R, Barbieri D, De Stefani A, Cellai E, Buglione M, Biti G (2012) A benchmark study on 883 nasopharyngeal cancer patients treated in two Italian Centres from 1977 to 2000. Part I: evolving technical choices and survival. Radiol Med 117:690–714
Magrini SM, Tonoli S, Costa L, Pasinetti P, Paiar F, Livi L, Simontacchi G, Meattini I, Pegurri L, Borghetti P, Frata P, Ponticelli P, Buglione M, Biti G (2012) A benchmark study on 883 nasopharyngeal cancer patients treated in two Italian Centres from 1977 to 2000. Part II: evolving technical choices and survival. Radiol Med 117:715–724
Perri F, Della Vittoria Scarpati G, Buonerba C, Di Lorenzo G, Longo F, Muto P, Schiavone C, Sandomenico F, Caponigro F (2013) Combined chemo-radiotherapy in locally advanced nasopharyngeal carcinomas. World J Clin Oncol 4(2):47–51
Orlandi E, Tomatis S, Potepan P, Bossi P, Mongioj V, Carrara M, Palazzi M, Franceschini M, Bergamini C, Locati L, Iannacone E, Guzzo M, Ibba T, Crippa F, Licitra L, Pignoli E, Fallai C (2013) Critical analysis of locoregional failures following intensity-modulated radiotherapy for nasopharyngeal carcinoma. Future Oncol 9(1):103–114
Moretto F, Rampino M, Munoz F, Ruo Redda MG, Reali A, Balcet V, Badellino S, Piva C, Schena M, Airoldi M, Ostellino O, Pecorari G, Ragona R, Ricardi U (2014) Conventional 2D (2DRT) and 3D conformal radiotherapy (3DCRT) versus intensity-modulated radiotherapy (IMRT) for nasopharyngeal cancer treatment. Radiol Med 119(8):634–641
Sobin LH, Witteking C (2002) UICC TNM classification of malignant tumours, 6th edn.
Grégoire V, Levendag P, Ang KK et al (2003) CT-Based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC, RTOG consensus guidelines. Radiother Oncol 69:227–236
The International Commission on Radiation Units and Measurements (2010) ICRU report n. 83, Prescribing, recording, and reporting photon-beam intensity-modulated Radiation Therapy (IMRT). J ICRU 10:1
Vermorken JB, Remenar E, van Herpen C et al (2007) Cisplatin, Fluoruracil and Docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704
Bossi P, Parolini D, Bergamini C, Locati LD, Orlandini E, Franceschini M (2009) TPF induction chemotherapy (CT) followed by concomitant cisplatin/radiotherapy (cCTRT) in locally advanced nasopharyngeal cancer (LANPC). J Clin Oncol 27. Abstract:6046
Ponzanelli A, Vigo V, Marcenaro M et al (2008) Induction chemotherapy followed by alternating chemo-radiotherapy in non endemic undifferentiated carcinoma of the nasopharynx: optimal compliance and promising 4‑year results. Oral Oncol 44:767–774
Baujat B, Audry H, Bourhis J, Chan A et al (2006) Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys 64:47–56
Blanchard P, Bourhis J et al (2013) Taxane-Cisplatin-Fluorouracil as induction chemotherapy in locally advanced head and Neck cancers: an individual patient data meta-analysis of the Meta-analysis of chemotherapy in head and neck cancer group. J Clin Oncol 31:2854–2860
Loong HH, Ma BBY, Leung S, Mo F et al (2012) Prognostic significance of the total dose of cisplatin administered during concurrent chemoradiotherapy in patients with locoregionally advanced nasopharyngeal carcinoma. Radiother Oncol 104:300–304
Kam MK, Wong FC, Kwong DL, Sze HC, Lee AW (2014) Current controversies in radiotherapy for nasopharyngeal carcinoma (NPC). Oral Oncol 50(10):907–912
Ho FC, Tham IW, Earnest A, Lee KM, Lu JJ (2012) Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 12:98
He X, Pan Z, Guo X, Ye M, Zhang Z, He S, Liu T (2012) The pattern of relapse and survival of elective irradiation of the upper neck for stage N0 nasopharyngeal carcinoma. J Radiat Oncol 7:35
Lee AWM, Henry S, Ng WT (2013) Is selective neck irradiation safe for node-negative nasopharyngeal carcinoma? Int J Radiat Oncol Biol Phys 85(4):902–903
Dirix P, Nuyts S (2010) Evidence-base organ-sparing radiotherapy in head and neck cancer. Lancet Oncol 11:85–91
Sun X, Su S, Chen C, Han F, Zhao C et al (2014) Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: An analysis of survival and treatment toxicities. Radiother Oncol 110(3):398–403
Acknowledgements
The authors thank the 136 patients who consented to release their data for the analysis. A particular acknowledgement to Dr. Filippo Bertoni for his help with statistical analyses and to Dr. Gundi Steinhilber for the German translation of the abstract.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Tonoli , D. Alterio, O. Caspiani, A. Bacigalupo, F. Bunkheila, M. Cianciulli, A. Merlotti, A. Podhradska, M. Rampino, D. Cante, L. Bruschieri, R. Gatta, and S.M. Magrini state that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Patients’ rights
the treatment given to patients was not experimental, representing the standard treatment in the single institution according to the national and international guidelines. Every patient gave informed consent for the treatment and the permission to use their data for this prospective observational analysis.
Ethical standards
The treatments given to the patients have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Additional information
Contribution author(s)
Study concept: Sandro Tonoli
Study design: Sandro Tonoli
Data acquisition: S. Tonoli, D. Alterio, O. Caspiani, A. Bacigalupo, F. Bunkheila, M. Cianciulli, A. Merlotti, A. Podhradska, M. Rampino, D. Cante, L. Bruschieri, S.M. Magrini
Quality control of data and algorithms: Sandro Tonoli, Roberto Gatta
Data analysis and interpretation: Sandro Tonoli
Statistical analysis: Sandro Tonoli
Manuscript preparation: S. Tonoli
Manuscript editing: S. Tonoli, D. Alterio, O. Caspiani, A. Bacigalupo, F. Bunkheila, M. Cianciulli, A. Merlotti, A. Podhradska, M. Rampino, D. Cante, L. Bruschieri, S.M. Magrini
Manuscript review: S. Tonoli, D. Alterio, O. Caspiani, A. Bacigalupo, F. Bunkheila, M. Cianciulli, A. Merlotti, A. Podhradska, M. Rampino, D. Cante, L. Bruschieri, S.M. Magrini
Names of principal investigators: S. Tonoli, D. Alterio, O. Caspiani, A. Bacigalupo, F. Bunkheila, M. Cianciulli, A. Merlotti, A. Podhradska, M. Rampino, D. Cante, L. Bruschieri, S.M. Magrini
Caption Electronic Supplementary Material
66_2016_1052_MOESM1_ESM.docx
Table 1: Number and percentage of cases treated with neo-adjuvant chemotherapy and/or concomitant chemotherapy according to the initial clinical stage (TNM 6th ed.)
Table 2: dose/fraction, number of fractions and total doses prescribed for high dose PTV
Table 3: Dose constraints for organs at risk
Table 4: dosimetric results description
Table 5: Radiotherapy overall treatment time (OTT: days) considering patients treated with alternated chemo-RT or not
Table 6: Influence of chemotherapy timing on the prolongation of radiotherapy overall treatment time
Rights and permissions
About this article
Cite this article
Tonoli, S., Alterio, D., Caspiani, O. et al. Nasopharyngeal carcinoma in a low incidence European area. Strahlenther Onkol 192, 931–943 (2016). https://doi.org/10.1007/s00066-016-1052-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-016-1052-2