Purpose:
To report on the clinical outcome of hypofractionated conformal radiotherapy (HCRT) for medically inoperable stage I non-small cell lung carcinoma (NSCLC) or limited pulmonary metastases ≤ 5 cm in diameter.
Patients and Methods:
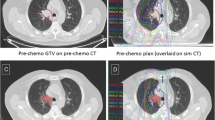
From June 2003 to March 2007, 40 patients (42 lesions) underwent HCRT consisting of 40 Gy in five fractions over 2.5 weeks received by at least 95% of planning target volume. All patients underwent CT simulation and treatment under free shallow breathing. To evaluate target displacement under respiratory activity, two additional CT scans were performed with breath-holding during the expiratory and inspiratory phases. Of all patients enrolled, those with a follow-up ≥ 4 months were considered suitable for analysis. Local response was evaluated with CT imaging 4 months after the end of HCRT and every 3 months thereafter. Local relapse-free survival (LRFS) and overall survival (OS) were calculated with the Kaplan-Meier method.
Results:
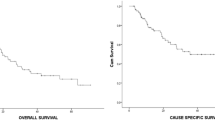
Local response to the treatment was complete response, partial response, no change, and progressive disease as seen in 29%, 43%, 14%, and 7% of tumors, respectively. LRFS at 1 year and 3 years was 76% and 63%, respectively. Lung toxicities ≥ grade 2 were observed in 4/40 patients, but no grade 4. Pericardial effusion occurred in one patient. In stage I NSCLC patients (n = 15) with a median follow-up of 25 months, the 1-year LRFS and OS rates were 88% and 81%, respectively, and the 3-year rates 72% and 61%, respectively.
Conclusion:
HCRT is an effective and low-toxic treatment for medically inoperable early-stage lung cancers and pulmonary metastases for all clinicians lacking the aid of a dedicated stereotactic system.
Ziel:
Klinische Auswertung einer hypofraktionierten konformalen Radiotherapie (HCRT) bei inoperablen nichtkleinzelligen Bronchialkarzinomen (NSCLC) im Stadium I oder isolierten Lungenmetastasen mit einem Durchmesser ≤ 5 cm.
Patienten und Methodik:
Von Juni 2003 bis März 2007 wurden 40 Patienten (42 Zielvolumina) einer CT-Simulation und HCRT (40 Gy in fünf Fraktionen uber 2,5 Wochen) von mindestens 95% des Planungszielvolumens unterzogen. In allen Fällen erfolgten die CT-Simulation und die Bestrahlung bei freier flacher Atmung. Zur Abschätzung der Zielvolumenverschiebung aufgrund der Atembewegung wurden zwei weitere CTs erstellt, während die Patienten den Atem jeweils in In- und Exspiration anhielten. Patienten mit einer Nachbeobachtungszeit von ≥ 4 Monaten wurden in die Studie eingeschlossen. Die lokale Ansprechrate wurde 4 Monate nach Behandlungsende und anschließend alle 3 Monate mittels CT ausgewertet. Lokalrezidivfreies Überleben (LRFS) und Gesamtüberleben (OS) wurden mit der Kaplan-Meier-Methode berechnet.
Ergebnisse:
Bezüglich des lokalen Therapieansprechens fanden sich Raten von 29%, 43%, 14% bzw. 7% für komplette Remission, partielle Remission, keine Veränderung bzw. Krankheitsprogression. Das 1- und das 3-Jahres-LRFS betrugen 76% und 63%. Lungentoxizitäten ≥ Grad 2 wurden bei 4/40 Patienten beobachtet, wobei allerdings keine Grad-4-Toxizitat auftrat. Bei einem Patienten kam es zu einem Perikarderguss. Bei NSCLC-Patienten im Stadium I (n = 15) mit einem durchschnittlichen Nachbeobachtungszeitraum von 25 Monaten betrugen die LRFS- und OS-Raten nach 1 Jahr 88% und 81% sowie nach 3 Jahren 72% und 61%.
Schlussfolgerung:
Die HCRT ist ein effektives und wenig toxisches Verfahren zur Behandlung inoperabler Patienten mit primären Lungenkarzinomen im Frühstadium und pulmonalen Metastasen.
Similar content being viewed by others
References
Aquino-Parsons C, Green A, Minchinton AI. Oxygen tension in primary gynaecological tumours: the influence of carbon dioxide concentration, Radiother Oncol 2000;57:45–51.
Auer F, Röper B, Scheich D, et al. Technical improvement of pO2 measurements in breast cancer: investigation of the feasability in patients an in vitro validation of the method. Strahlenther Onkol 2007;183:265–70.
Brizel DM, Dodge RK, Clough RW, et al. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol 1999;53:113–7.
Chen Q, Andersen AH, Zhang Z, et al. Mapping drug-induced changes in cerebral R2* by multiple gradient recalled echo functional MRI. Magn Reson Imaging 1996;14:469–76.
Dellas K, Bache M, Pigorsch SU, et al. Prognostic impact of HIF-1α expression in patients with definitive radiotherapy for cervical cancer. Strahlenther Onkol 2008;184:169–74.
Diergarten T, Martirosian P, Kottke R, et al. Functional characterization of prostate cancer by integrated magnetic resonance imaging and oxygenation changes during carbogen breathing. Invest Radiol 2005;40:102–9.
Di Martino EF, Gagel B, Schramm O, et al. Evaluation of tumor oxygenation by color duplex sonography: a new approach. Otolaryngol Head Neck Surg 2005;132:765–9.
Dunn TJ, Braun RD, Rhemus WE, et al. The effects of hyperoxic and hypercapnic gases on tumour blood flow. Br J Cancer 1999;80:117–26.
Dunn JF, O’Hara JA, Zaim-Wadghiri Y, et al. Changes in oxygenation of intracranial tumors with carbogen: a BOLD MRI and EPR oximetry study. J Magn Reson Imaging 2002;16:511–21.
Duyn JH, Moonen CT, van Yperen GH, et al. Inflow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echoes at 1.5 T. NMR Biomed 1994;7:83–8.
Fyles AW, Milosevic M, Wong R, et al. Oxygenation predicts radiation response and survival in patients with cervix cancer. Radiother Oncol 1998;48:149–56.
Gagel B, Di Martino E, Schramm O, et al. Contrast-enhanced color duplex sonography (CDS): an alternative for the evaluationor therapy-relevant tumor oxygenation? Strahlenther Onkol 2006;182:604–9.
Gillies RJ, Raghunand N, Karczmar GS, et al. MRI of the tumor microenvironment. J Magn Reson Imaging 2002;16:430–50.
Gillies RJ, Schornack PA, Secomb TW, et al. Causes and effects of heterogeneous perfusion in tumors. Neoplasia 1999;1:197–207.
Gray LH, Conger AD, Ebert M, et al. The concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638–48.
Hickham JB, Frayser R. Studies of the retinal circulation in man: observations in vessel diameter, arteriovenous oxygen difference and mean circulation time. Circulation 1966;33:302–16.
Kaanders JH, Bussink J, van der Kogel AJ. ARCON: a novel biology-based approach in radiotherapy. Lancet Oncol 2002;3:728–37.
Kappler M, Taubert H, Holzhausen HJ, et al. Immunohistochemical detection of HIF-1alpha and CAIX in advanced head-and-neck cancer. Prognostic role and correlation with tumor markers and tumor oxygenation parameters. Strahlenther Onkol 2008;184:393–9. (Erratum in: Strahlenther Onkol 2008;184:491.)
Kim SG, Hendrich K, Hu X, et al. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed 1994;7: 69–74.
Kinoshita Y, Kohshi K, Kunugita N, et al. Preservation of tumour oxygen after hyperbaric oxygenation monitored by magnetic resonance imaging. Br J Cancer 2000;82:88–92.
Knocke TH, Weitmann HD, Feldmann HJ, et al. Intratumoral pO2-measurements as predictive assay in the treatment of carcinoma of the uterine cervix. Radiother Oncol 1999;53:99–104.
Lanzen JL, Braun RD, Ong AL, et al. Variability in blood flow and pO2 in tumors in response to carbogen breathing. Int J Radiol Biol Phys 1998;42:855–9.
Lövey J, Bereczky B, Gilly R, et al. Recombinant human erythropoietin alpha improves the efficacy of radiotherapy of a human tumor xenograft, affecting tumor cells and microvessels. Strahlenther Onkol 2008;184: 1–7.
Lyng, H, Tanum G, Evensen JF, et al. Changes in oxygen tension during radiotherapy of head and neck tumours. Acta Oncol 1999;38:1037–42.
Lyng H, Vorren AO, Sundfor K, et al. Intra- and intertumor heterogeneity in blood perfusion of human cervical cancer before treatment and after radiotherapy. Int J Cancer 2001;96:182–90.
Matsumoto K, Bernardo M, Subramanian S, et al. MR assessment of changes of tumor in response to hyperbaric oxygen treatment. Magn Reson Med 2006;56:240–6.
McSheehy PM, Port RE, Rodrigues LM, et al. Investigations in vivo of the effects of carbogen breathing on 5-fluorouracil pharmacokinetics and physiology of solid rodent tumours. Cancer Chemother Pharmacol 2005;55: 117–28.
Menon RS, Ogawa S, Tank DW, et al. Tesla gradient recalled echo characteristics of photic stimulation-induced signal changes in the human primary visual cortex. Magn Reson Med 1993;30:380–6.
Nordsmark M, Overgaard J. A confirmatory prognostic study on oxygenation status and loco-regional control in advanced head and neck squamous cell carcinoma treated by radiation therapy. Radiother Oncol 2000;57:39–43.
Ogawa S, Lee TM, Kay AR, et al. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A 1990;87:9868–72.
Ogawa S, Lee TM, Nayak AS, et al. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 1990;14:68–78.
Pakola SJ, Grunwald JE. Effects of oxygen and carbon dioxide on human retinal circulation. Invest Ophthalmol Vis Sci 1993;34:2866–70.
Powell ME, Collingridge DR, Saunders MI, et al. Improvement in human tumour oxygenation with carbogen of varying carbon dioxide concentrations. Radiother Oncol 1999;50:167–71.
Prasad PV, Chen Q, Goldfarb JW, et al. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging 1997;7:1163–5.
Rijpkema M, Kaanders JH, Schuuring J, et al. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head and neck tumors as measured by magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2002;53:1185–91.
Rijpkema M, Schuuring J, Bernsen PL, et al. BOLD MRI response to hypercapnic hyperoxia in patients with meningiomas: correlation with gadolinium-DTPA uptake rate. Magn Reson Imaging 2004;22:761–7.
Robinson SP, Rodrigues LM, Howe FA, et al. Effects of different levels of hypercapnic hyperoxia on tumour R(2)* and arterial blood gases. Magn Reson Imaging 2001;19:161–6.
Rudat V, Vanselow B, Wollensack P, et al. Repeatability and prognostic impact of the pretreatment pO2 histography in patients with advanced head and neck cancer. Radiother Oncol 2000;57:31–7.
Schmitt P, Kotas M, Tobermann A, et al. Quantitative tissue perfusion measurements in head and neck carcinoma patients before and during radiation therapy with a non-invasive MR imaging spin-labeling technique. Radiother Oncol 2003;67:27–34.
Schuuring J, Bussink J, Bernsen H, et al. Effect of carbogen breathing on the radiation response of a human glioblastoma xenograft. Strahlenther Onkol 2006;182:408–14.
Schuuring J, Rijpkema M, Bernsen H, et al. Effect of breathing a hyperoxic hypercapnic gas mixture on the oxygenation of meningiomas; preliminary results. J Neurooncol 2002;57:127–32.
Thews O, Keleher D, Vaupel P. Dynamics of tumor oxygenation and red blood cell flux in response to inspiratory hyperoxia combined with different levels of inspiratory hypercapnia. Radiother Oncol 2002;62:77–85.
Van der Sanden BP, Heerschap A, Hoofd L, et al. Effect of carbogen breathing on the physiological profile of human glioma xenografts. Magn Reson Med 1999;42:490–9.
Vaupel P, Dunst J, Engert A, et al. Effects of recombinant human erythropoietin (rHuEPO) on tumor control in patients with cancer-induced anemia. Onkologie 2005;28:216–21.
Zips D, Adam M, Flentje M, et al. Impact of hypoxia and the metabolic microenvironment on radiotherapy of solid tumors. Introduction of a multiinstitutional research Project. Strahlenther Onkol 2004;180:609–15.
Zips D, Yaromina A, Schütze C, et al. Experimental evaluation of functional imaging for radiotherapy. Strahlenther Onkol 2007;183:Spec No 2:41–2.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mirri, M.A., Arcangeli, G., Benassi, M. et al. Hypofractionated Conformal Radiotherapy (HCRT) for Primary and Metastatic Lung Cancers with Small Dimension. Strahlenther Onkol 185, 27–33 (2009). https://doi.org/10.1007/s00066-009-1873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-009-1873-3