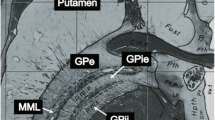
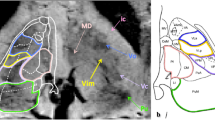
Abstract
Objective
Deep brain stimulation has received increasing attention in recent years as a treatment option for many neurological diseases. The thalamic nuclei in particular are widely used as targets. The goal of the present work was to evaluate whether the combination of two known MRI techniques can lead to identification of thalamic substructures at 3 T.
Methods
In nine healthy subjects, an optimized 3D magnetization prepared rapid acquisition GRE (MPRAGE) protocol and phase data from a 3D GRE sequence were combined to form a new contrast (MPRAGE*). The depiction of 13 thalamic substructures was rated by two independent raters in the MPRAGE, phase and MPRAGE* image on a five-point scale. Inter-rater reliability was scored with a weighted Cohen’s kappa.
Results
Inter-rater reliability was good, with the average weighted κ = 0.68. No significant difference between the depiction of the thalamic substructures between phase and MPRAGE images could be found. MPRAGE* showed a significantly better depiction of thalamic substructures in comparison to MPRAGE and phase (p < 0.001 for both cases).
Conclusion
The combination of an optimized MPRAGE protocol with phase data to form an MPRAGE* image leads to a further improvement in the depiction of thalamic substructures, which enables the depiction of thalamic nuclei at 3 T.





Similar content being viewed by others
References
Chen XL, Xiong YY, Xu GL, Liu XF. Deep brain stimulation. Interv Neurol. 2013;1:200–212.
Benabid AL, Pollak P, Hoffmann D, Gao DM, Hommel M, Perret JE, Rougermont J de. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406.
Weiss D, Mielke C, Wächter T, Bender B, Liscic RM, Scholten M, Naros G, Pewnia C, Gharabaghi A, Krüger R. Long-term outcome of deep brain stimulation in fragile X‑associated tremor/ataxia syndrome. Parkinsonism Relat Disord. 2015;21:310–313.
Yamamoto T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, Tsubokawa T. Deep brain stimulation for the treatment of vegetative state. Eur J Neurosci. 2010;32:1145–1151.
Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, Oommen K, Osorio I, Nazzaro J, Labar D, Kaplitt M, Sperling M, Sandok E, Neal J, Handforth A, Stern J, DeSalles A, Chung S, Shetter A, Bergen D, Bakay R, Henderson J, French J, Baltuch G, Rosenfeld W, Youkilis A, Marks W, Garcia P, Barbaro N, Fountain N, Bazil C, Goodman R, McKhann G, Babu Krishnamurthy K, Papavassiliou S, Epstein C, Pollard J, Tonder L, Grebin J, Coffey R, Graves N, SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refactory epilepsy. Epilepsia. 2010;51:899–908.
Sartorius A, Kiening KL, Kirsch P, Gall CC von, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11.
Ackermans L, Duits A, Linden C van der, Tijssen M, Schruers K, Temel Y, Kleijer M, Nederveen P, Bruggeman R, Tromp S, Kranen-Mastenbroek V van, Kingma H, Cath D, Visser-Vandewalle V. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain. 2011;134:832–844.
Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, Fritz B, Eisenberg B, Biondi T, O’Connor J, Kobylarz EJ, Farris S, Machado A, McCagg C, Plum F, Fins JJ, Rezai AR. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–604.
Deoni SCL, Josseau MJC, Rutt BK, Peters TM. Visualization of thalamic nuclei on high resolution, multi-averaged T1 and T2 maps acquired at 1.5 T. Hum Brain Mapp. 2005;25:353–359.
Gringel T, Schulz-Schaeffer W, Elolf E, Frölich A, Dechent P, Helms G. Optimized high-resolution mapping of magnetization transfer (MT) at 3 Tesla for direct visualization of substructures of the human thalamus in clinically feasible measurement time. J Magn Reson Imaging. 2009;29:1285–1292.
Bender B, Mänz C, Korn A, Nägele T, Klose U. Optimized 3D Magnetization-Prepared Rapid Acquisition of Gradient Echo: Identification of Thalamus Substructures at 3 T. AJNR Am J Neuroradiol. 2011;32:2110–2115.
Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK. Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7 T. Neuroimage. 2014;84:534–545.
Abosch A, Yacoub E, Ugurbil K, Harel N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 tesla. Neurosurgery. 2010;67:1745–1756.
Deistung A, Schäfer A, Schweser F, Biedermann U, Turner R, Reichenbach JR. Toward in vivo histology: a comparison of quantitative susceptibility mapping (QSM) with magnitude-, phase-, and R2*-imaging at ultra-high magnetic field strength. Neuroimage. 2013;65:299–314.
Lemaire JJ, Sakka L, Ouchchane L, Caire F, Gabrillarques J, Bonny JM. Anatomy of the human thalamus based on spontaneous contrast and microscopic voxels in high-field magnetic resonance imaging. Neurosurgery. 2010;66(3 Suppl Operative):161–172.
Walsh DO, Gmitro AF, Marcellin MW. Adaptive reconstruction of phased array MR imagery. Magn Reson Med. 2000;43:682–690.
Noll DC, Nishimura DG, Macovski A. Homodyne detection in magnetic resonance imaging. IEEE Trans Med Imaging. 1991;10:154–163.
Nelles M, Koenig R, Kandyba J, Schaller C, Urbach H. Fusion of MRI and CT with subdural grid electrodes. Zentralbl Neurochir. 2004;65:174–179.
Morel A, Magnin M, Jeanmond D. Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol. 1997;387:588–630.
Schaltenbrand G, Wahren W. Atlas for Stereotaxy of the human brain. Stuttgart: Georg Thieme Verlag; 1991.
Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220.
Altman DG. Practical statistics for medical research. Boca Raton: Taylor & Francis; 1990.
Saranathan M, Tourdias T, Bayram E, Pejman G, Rutt KR. Optimization of white-matter-nulled magnetization prepared rapid gradient echo (MP-RAGE) imaging. Magn Reson Med. 2015;73:1786–1794.
Haacke E, Liu S, Buch S, Zheng W, Wu D, Ye Y. Quantitative susceptibility mapping: current status and future directions. Magn Reson Imaging. 2015;33:1–25.
Stortmann B, Heidemann RM, Anwander A, Weiss M, Trampel R, Villringer A, Turner R. High-resolution MRI and diffusion-weighted imaging of the human habenula at 7 tesla. J Magn Reson Imaging. 2014;39:1018–1026.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B. Bender, S. Wagner and U. Klose state that there are no conflicts of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Bender, B., Wagner, S. & Klose, U. Optimized depiction of thalamic substructures with a combination of T1-MPRAGE and phase: MPRAGE*. Clin Neuroradiol 27, 511–518 (2017). https://doi.org/10.1007/s00062-016-0513-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-016-0513-4