Abstract
Purpose
The authors investigated the potential of a 32-channel (32ch) receiving head coil for functional magnetic resonance imaging (fMRI) compared to a standard eight-channel (8ch) coil using a motor task.
Material and Methods
Brain activation was analyzed in 14 healthy right-handed subjects performing finger tapping with the right index finger (block design) during two experimental sessions, one with the 8ch and one with the 32ch coil (applied in a pseudorandomized order). Additionally, a phantom study was performed to compare signal-to-noise ratios (SNRs) of both coils.
Results
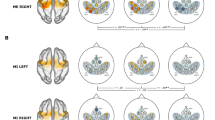
During both fMRI sessions, analysis of motor conditions resulted in an activation of the left “hand knob” (precentral gyrus). Application of the 32ch coil obtained additional activation clusters in the right cerebellum, left superior frontal gyrus (SMA), left supramarginal gyrus, and left postcentral gyrus. The phantom study revealed a significantly higher SNR for the 32ch coil compared to the 8ch coil in superficial cortical areas located near the surface of the brain.
Conclusion
The 32ch technology has a potential impact on fMRI studies, especially in paradigms that result in activation of cortical areas located near the surface of the brain.
Zusammenfassung
Ziel
In diesem Artikel wurde der Einfluss einer 32-Kanal-Empfangsspule im Vergleich zu einer Acht-Kanal-Standardspule auf die Ergebnisse einer funktionellen Magnetresonanztomographie-(fMRT-)Studie, in der ein motorisches Paradigma genutzt wurde, untersucht.
Material und Methodik
Die Hirnaktivierung von 14 gesunden Rechtshändern, die eine Fingertapping-Aufgabe mit dem rechten Zeigefinger (Blockdesign) ausführten, wurde während zweier experimenteller Durchläufe in pseudorandomisierter Abfolge untersucht. Während eines Durchlaufs wurde eine 32-Kanal-Spule, während eines zweiten Durchlaufs eine Acht-Kanal-Spule benutzt. Außerdem wurde eine Phantomstudie durchgeführt, um das Signal-Rausch-Verhältnis beider Empfangsspulen zu vergleichen.
Ergebnisse
Die Analyse der Motorbedingung während beider Durchläufe resultierte in einer Aktivierung des linken Handareals (Gyrus praecentralis). Die Verwendung der 32-Kanal-Spule resultierte in einer Aktivierung von zusätzlichen Hirnarealen wie dem rechten Cerebellum, linken Gyrus frontalis superior (SMA), linken Gyrus supramarginalis und linken Gyrus postcentralis. Die Phantomstudie zeigte ein signifikant höheres Signal-Rausch-Verhältnis in Arealen nahe der Hirnoberfläche für die 32-Kanal-Spule im Vergleich zur Acht-Kanal-Spule.
Schlussfolgerung
Die 32-Kanal-Empfangsspulen-Technologie hat einen potentiellen Einfluss auf die Ergebnisse von fMRT-Studien, im Speziellen auf Paradigmen, die in Hirnaktivierung von kortikalen Arealen nahe der Hirnoberfläche resultieren.



Similar content being viewed by others
References
Otazo R, Tsai SY, Lin FH, Posse S. Accelerated short-TE 3D proton echo-planar spectroscopic imaging using 2D-SENSE with a 32-channel array coil. Magn Reson Med. 2007;58:1107–16.
Tsai SY, Otazo R, Posse S, Lin YR, Chung HW, Wald LL, Wiggins GC, Lin FH. Accelerated proton echo planar spectroscopic imaging (PEPSI) using GRAPPA with a 32-channel phased-array coil. Magn Reson Med. 2008;59:989–98.
Wiggins GC, Triantafyllou C, Potthast A, Reykowski A, Nittka M, Wald LL. 32-channel 3 Tesla receive-only phased-array head coil with soccer-ball element geometry. Magn Reson Med. 2006;56:216–23.
Zhu Y, Hardy CJ, Sodickson DK, Giaquinto RO, Dumoulin CL, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Ohliger MA, Rofsky NM. Highly parallel volumetric imaging with a 32-element RF coil array. Magn Reson Med. 2004;52:869–77.
McDougall MP, Wright SM. 64-channel array coil for single echo acquisition magnetic resonance imaging. Magn Reson Med. 2005;54:386–92.
de Zwart JA, Ledden PJ, van Gelderen P, Bodurka J, Chu R, Duyn JH. Signal-to-noise ratio and parallel imaging performance of a 16-channel receive-only brain coil array at 3.0 Tesla. Magn Reson Med. 2004;51:22–6.
McDougall MP, Wright SM. Investigation of coil phase compensation in 3D imaging at very high acceleration factors. J Magn Reson Imaging. 2007;25:1305–11.
Sodickson DK, Hardy CJ, Zhu Y, Giaquinto RO, Gross P, Kenwood G, Niendorf T, Lejay H, McKenzie CA, Ohliger MA, Grant AK, Rofsky NM. Rapid volumetric MRI using parallel imaging with order-of-magnitude accelerations and a 32-element RF coil array: feasibility and implications. Acad Radiol. 2005;12:626–35.
Hardy CJ, Darrow RD, Saranathan M, Giaquinto RO, Zhu Y, Dumoulin CL, Bottomley PA. Large field-of-view real-time MRI with a 32-channel system. Magn Reson Med. 2004;52:878–84.
Porter JR, Wright SM, Reykowski A. A 16-element phased-array head coil. Magn Reson Med. 1998;40:272–9.
Schoth F, Kraemer N, Niendorf T, Hohl C, Gunther RW, Krombach GA. Comparison of image quality in magnetic resonance imaging of the knee at 1.5 and 3.0 Tesla using 32-channel receiver coils. Eur Radiol. 2008;18:2258–64.
Bodurka J, Ledden PJ, van Gelderen P, Chu R, de Zwart JA, Morris D, Duyn JH. Scalable multichannel MRI data acquisition system. Magn Reson Med. 2004;51:165–71.
de Zwart JA, van Gelderen P, Kellman P, Duyn JH. Application of sensitivity-encoded echo-planar imaging for blood oxygen level-dependent functional brain imaging. Magn Reson Med. 2002;48:1011–20.
de Zwart JA, van Gelderen P, Kellman P, Duyn JH. Reduction of gradient acoustic noise in MRI using SENSE-EPI. Neuroimage. 2002;16:1151–5.
Frederick Bd, Wald LL, Maas LC 3rd, Renshaw PF. A phased array echoplanar imaging system for fMRI. Magn Reson Imaging. 1999;17:121–9.
Hesselmann V, Girnus R, Wedekind C, Hunsche S, Bunke J, Schulte O, Sorger B, Lasek K, Krug B, Sturm V, Lackner K. Functional MRI using multiple receiver coils: BOLD signal changes and signal-to-noise ratio for three-dimensional-PRESTO vs. single shot EPI in comparison to a standard quadrature head coil. J Magn Reson Imaging. 2004;20:321–6.
Kim SG, Tsekos NV, Ashe J. Multi-slice perfusion-based functional MRI using the FAIR technique: comparison of CBF and BOLD effects. NMR Biomed. 1997;10:191–6.
Brooks BR, Bushara K, Khan A, Hershberger J, Wheat JO, Belden D, Henningsen H. Functional magnetic resonance imaging (fMRI) clinical studies in ALS—paradigms, problems and promises. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1 Suppl 2:S23–32.
Lee HK, Kim JS, Hwang YM, Lee MJ, Choi CG, Suh DC, Lim TH. Location of the primary motor cortex in schizencephaly. AJNR Am J Neuroradiol. 1999;20:163–6.
Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, DeLaPaz RL. Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke. 2000;31:656–61.
Chen XK, Xiao YY, Zheng WB, Chen FY, Wu RH. Functional magnetic resonance mapping of motor cortex in patients with mass lesions near primary motor and sensory cortices. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1877–80.
Gordon AM, Lee JH, Flament D, Ugurbil K, Ebner TJ. Functional magnetic resonance imaging of motor, sensory, and posterior parietal cortical areas during performance of sequential typing movements. Exp Brain Res. 1998;121:153–66.
Kawashima R, Itoh H, Ono S, Satoh K, Furumoto S, Gotoh R, Koyama M, Yoshioka S, Takahashi T, Takahashi K, Yanagisawa T, Fukuda H. Changes in regional cerebral blood flow during self-paced arm and finger movements. A PET study. Brain Res. 1996;716:141–8.
Habas C, Axelrad H, Cabanis EA. The cerebellar second homunculus remains silent during passive bimanual movements. Neuroreport. 2004;15:1571–4.
Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113.
Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210.
Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–55.
Brett M, Penny WD, Kiebel S. An introduction to random field theory. In: Frackowiak RSJ, Ashburner JT, Penny WD, Zeki S, Friston KJ, Frith CD, Dolan RJ, Price CJ, editors. Human brain function. 2nd ed. San Diego: Elsevier; 2004. p. 867–879.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–35.
Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. Amsterdam: Elsevier Academic Press; 2004.
Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–57.
van Gelderen P, Ramsey NF, Liu G, Duyn JH, Frank JA, Weinberger DR, Moonen CT. Three-dimensional functional magnetic resonance imaging of human brain on a clinical 1.5-T scanner. Proc Natl Acad Sci U S A. 1995;92:6906–10.
Rao SM, Binder JR, Bandettini PA, Hammeke TA, Yetkin FZ, Jesmanowicz, A, Lisk LM, Morris GL, Mueller WM, Estkowski LD, Wong EC, Haughton VM, Hyde JS. Functional magnetic resonance imaging of complex human movements. Neurology. 1993;43:2311–8.
Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–36.
Nakai T, Kato C, Glover GH, Toma K, Moriya T, Matsuo K. A functional magnetic resonance imaging study of internal modulation of an external visual cue for motor execution. Brain Res. 2003;968:238–47.
Rubia K, Overmeyer S, Taylor E, Brammer M, Williams S, Simmons A, Andrew C, Bullmore E. Prefrontal involvement in “temporal bridging” and timing movement. Neuropsychologia. 1998;36:1283–93.
Tieleman A, Deblaere K, Van Roost D, Van Damme O, Achten E. Preoperative fMRI in tumour surgery. Eur Radiol. 2009;19:2523–34.
Chakraborty A, McEvoy AW. Presurgical functional mapping with functional MRI. Curr Opin Neurol. 2008;21:446–51.
Moller M, Freund M, Greiner C, Schwindt W, Gaus C, Heindel W. Real time fMRI: a tool for the routine presurgical localisation of the motor cortex. Eur Radiol. 2005;15:292–5.
Haller S, Bartsch AJ. Pitfalls in fMRI. Eur Radiol. 2009;19:2689–706.
Conflict of Interest Statement
The authors declare that there is no actual or potential conflict of interest in relation to this article.
The 8ch and the 32ch coil were provided by GE Healthcare, Solingen, Germany. The authors who were not employees of GE Healthcare had full control of inclusion of any data and information that might have presented a conflict of interest for those authors who were employees of GE Healthcare at every time point of the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Albrecht, J., Burke, M., Haegler, K. et al. Potential Impact of a 32-Channel Receiving Head Coil Technology on the Results of a Functional MRI Paradigm. Clin Neuroradiol 20, 223–229 (2010). https://doi.org/10.1007/s00062-010-0029-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-010-0029-2