Abstract
Objectives
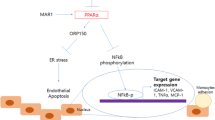
Endothelial dysfunction is involved in various aspects of vascular biology and different stages of cardiovascular diseases (CVDs). Nucleotide-binding oligomerization domain-containing protein (NOD) 2, a pivotal innate immune receptor for muramyl dipeptide (MDP), has been reported to be a central regulator in CVDs. Previously, we reported that NOD2 played a leading role in MDP-triggered oxidative stress in endothelial cells (ECs). However, whether NOD2 participates in the regulatory mechanism of vascular cell adhesion molecule‑1 (VCAM-1) and endothelin‑1 (ET-1) expression was not elucidated.
Methods
Human umbilical vein endothelial cells (HUVECs) were stimulated with MDP for 12 h. mRNA expression of VCAM‑1 and ET‑1 was detected using real time polymerase chain reaction (PCR). Scrambled control small interfering RNA (siRNA) and NOD2 siRNA were transfected into HUVECs using Lipofectamine 2000 reagent (Invitrogen, Waltham, MA, USA). Furthermore, pyrrolidine dithiocarbamate was adopted to investigate the effect of nuclear factor κB (NF-κB) on NOD2-mediated VCAM‑1 and ET‑1 gene expression in MDP-treated HUVECs.
Results
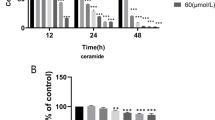
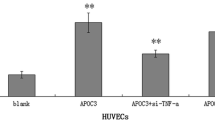
Data showed that MDP significantly increased VCAM‑1 and ET‑1 mRNA expression, which was dependent on NOD2. In addition, NF-κB inhibition suppressed NOD2-mediated gene expression of VCAM‑1 and ET‑1.
Conclusion
Collectively, we confirmed NOD2 aggravated VCAM‑1 and ET‑1 gene expression through NF-κB in HUVECs treated with MDP.
Zusammenfassung
Zielsetzungen
Eine endotheliale Dysfunktion ist an vielfältigen Aspekten der Gefäßbiologie und an unterschiedlichen Stadien von Herz-Kreislauf-Erkrankungen (CVDs) beteiligt. NOD2 („nucleotide-binding oligomerization domain-containing protein“), ein entscheidender angeborener Immunrezeptor für Muramyldipeptid (MDP), wurde als zentraler Regulator bei Herz-Kreislauf-Erkrankungen beschrieben. In früheren Veröffentlichungen haben wir dargelegt, dass NOD2 eine wesentliche Funktion beim MDP-getriggerten oxidativen Stress in Endothelzellen (ECs) hat. Ob NOD2 jedoch am Regulationsmechanismus der Expression von VCAM‑1 („vascular cell adhesion molecule-1“) und ET‑1 („endothelin-1“) beteiligt ist, ließ sich nicht eindeutig klären.
Methoden
Humane Nabelvenenendothelzellen (HUVECs) wurden 12 h lang mit MDP stimuliert. Wir konnten die mRNA-Expression von VCAM‑1 und ET‑1 mittels Real-time-PCR (Polymerasekettenreaktion) nachweisen. „Scrambled control“ siRNA („small interfering RNA“) und NOD2-siRNA wurden mit Lipofectamine 2000-Reagenz (Invitrogen, Waltham/MA, USA) in HUVECs transfiziert. Ferner wurde Pyrrolidindithiocarbamat eingesetzt, um die Wirkung des nukleären Faktors κB (NF-κB) auf die NOD2-vermittelte VCAM-1- und ET-1-Genexpression in MDP-vorbehandelten HUVECs zu untersuchen.
Ergebnisse
Die Befunde zeigten, dass MDP die von NOD2 abhängige VCAM-1- und ET-1-mRNA-Expression signifikant erhöhte. Darüber hinaus supprimerte die NF-κB-Inhibition die NOD2-vermittelte Genexpression von VCAM‑1 und ET‑1.
Schlussfolgerung
Insgesamt bestätigten wir die durch NOD2 beeinträchtigte VCAM-1- und ET-1-Genexpression mittels NF-κB in mit MDP vorbehandelten HUVECs.




Similar content being viewed by others
References
Saeedf A, Dabhadkar K, Virani SS et al (2018) Cardiovascular disease prevention: training opportunities, the challenges, and future directions. Curr Atheroscler Rep 20(7):35
Ma LY, Wang W, Wu YZ et al (2018) A12514 outline of the report on cardiovascular diseases in China. J Hypertens 36:e322
Chen L, Kong LJ, Wei XB et al (2019) β‑arrestin 2 negatively regulates NOD2 signalling pathway through association with TRAF6 in microglia after cerebral ischaemia/reperfusion injury. J Cell Mol Med 23(5):3325–3335
Stefano DA, Ricciardolo FLM, Caramori G et al (2017) Bronchial inflammation and bacterial load in stable COPD is associated with TLR4 overexpression. Eur Respir J 49(5):1602006
Liu HQ, Zhang XY, Edfeldt K et al (2013) NOD2-mediated innate immune signaling regulates the eicosanoids in atherosclerosis. Arterioscler Thromb Vasc Biol 33:2193–2201
Kong LJ, Liu XQ, Xue Y et al (2018) Muramyl dipeptide induces reactive oxygen species generation through the NOD2/COX-2/NOX4 signaling pathway in human umbilical vein endothelial cells. J Cardiovasc Pharmacol 71(6):352–358
Huiqing L, Xinbing W, Lingjun K et al (2015) NOD2 is involved in the inflammatory response after cerebral ischemia-reperfusion injury and triggers NADPH oxidase 2‑derived reactive oxygen species. Int J Biol Sci 11(5):525–535
Johansson ME, Zhang XY, Edfeldt K et al (2014) Innate immune receptor NOD2 promotes vascular inflammation and formation of lipid-rich necrotic cores in hypercholesterolemic mice. Eur J Immunol 44(10):3081–3092
Ohlsson C, Nigro G, Boneca IG et al (2017) Regulation of bone mass by the gut microbiota is dependent on NOD1 and NOD2 signaling. Cell Immunol 317:55–58
Witkowski M, Landmesser U, Rauch U (2016) Tissue factor as a link between inflammation and coagulation. Trends Cardiovasc Med 26(4):297–303
van Hinsbergh VWM (2012) Endothelium-role in regulation of coagulation and inflammation. Semin Immunopathol 34(1):93–106
Chen YW, Apostolakis S, Lip GYH (2014) Exercise-induced changes in inflammatory processes: implications for thrombogenesis in cardiovascular disease. Ann Med 46(7):439–455
Habas K, Shang LJ (2018) Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 54:139–143
Pober JS, Sessa WC (2015) Inflammation and the blood microvascular system. Cold Spring Harb Perspect Biol 7(1):a16345
Rostampour N, Fekri K, Hashemi-Dehkordi E et al (2017) Association between vascular endothelial markers and carotid intima-media thickness in children and adolescents with type 1 diabetes mellitus. J Clin Diagn Res 11(9):SC1–SC5
Peter K, Weirich U, Nordt TK et al (1999) Soluble vascular cell adhesion molecule‑1 (VCAM-1) as potential marker of atherosclerosis. Thromb Haemost 82(S01):38–43
El-Ashmawy HM, Selim FO, Hosny TAM et al (2019) Association of low serum meteorin like (Metrnl) concentrations with worsening of glucose tolerance, impaired endothelial function and atherosclerosis. Diabetes Res Clin Pract 150:57–63
Di Franco M, Lucchino B, Conti F et al (2018) Asymmetric dimethyl arginine as a biomarker of atherosclerosis in rheumatoid arthritis. Mediators Inflamm 2018:ID3897295
Houde M, Desbiens L, D’Orléans-Juste P (2016) Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol 77:143–175
Kowalczyk A, Kleniewska P, Kolodziejczyk M et al (2015) The role of endothelin‑1 and endothelin receptor antagonists in inflammatory response and sepsis. Arch Immunol Ther Exp 63(1):41–52
Jackson AO, Regine MA, Subrata C et al (2018) Molecular mechanisms and genetic regulation in atherosclerosis. Int J Cardiol Heart Vasc 21:36–44
Gopalakrishna D, Pennington S, Karaa A et al (2016) ET‑1 stimulates superoxide production by eNOS following exposure of vascular endothelial cells to endotoxin. Shock 46(1):60–66
Oh HM, Lee HJ, Seo GS et al (2005) Induction and localization of NOD2 protein in human endothelial cells. Cell Immunol 237(1):37–44
Yurdagul A, Sulzmaier FJ, Chen XL et al (2016) Oxidized LDL induces FAK-dependent RSK signaling to drive NF-κB activation and VCAM‑1 expression. J Cell Sci 129(8):1580–1591
Girardin SE, Boneca IG, Viala J et al (2003) NOD2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278:8869–8872
Rubanyi GM (1993) The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol 22:S1–14
Haynes WG, Webb DJ (1998) Endothelin as a regulator of cardiovascular function in health and disease. J Hypertens 16(8):1081–1098
Haybar H, Shahrabi S, Rezaeeyan H et al (2019) Endothelial cells: from dysfunction mechanism to pharmacological effect in cardiovascular disease. Cardiovasc Toxicol 19(1):13–22
Xin Z, Chonghuai G, Chenghui Y et al (2016) NALP3-inflammasome-related gene polymorphisms in patients with prehypertension and coronary atherosclerosis. Biomed Res Int 2016:1–10
Ting JP, Duncan JA, Lei Y (2010) How the noninflammasome NLRs function in the innate immune system. Science 327:286–290
Henseleit U, Steinbrink K, Sunderkötter C et al (1994) Expression of murine VCAM‑1 in vitro and in different models of inflammation in vivo: correlation with immigration of monocytes. Exp Dermatol 3(6):249–256
Javeshghani D, Barhoumi T, Idris-Khodja N et al (2013) Reduced macrophage-dependent inflammation improves endothelin-1-induced vascular injury. Hypertension 62(1):112–117
Idriskhodja N, Ouerd S, Trindade M et al (2017) Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens 35(7):1390–1401
Sofiane O, Noureddine IK, Rehman MMO et al (2016) Lbps 01-03 endothelin‑1 overexpression exaggerates diabetes-induced endothelial dysfunction by altering oxidative stress balance. J Hypertens 34:e174
Liu J, Wang Y, Ouyang X (2014) Beyond toll-like receptors: porphyromonas gingivalis induces IL‑6, IL‑8, and VCAM‑1 expression through NOD-mediated NF-κB and ERK signaling pathways in periodontal fibroblasts. Inflammation 37(2):522–533
Duerrschmidt N, Wippich N, Goettsch W et al (2000) Endothelin‑1 induces NAD(P)H oxidase in human endothelial cells. Biochem Biophys Res Commun 269(3):713–717
Jiang Y, Jiang LLI, Maimaitirexiati XMZY et al (2015) Irbesartan attenuates TNF-α-induced ICAM‑1, VCAM‑1, and E‑selectin expression through suppression of NF-κB pathway in HUVECs. Eur Rev Med Pharmacol Sci 19(17):3295–3302
Addabbo F, Nacci C, Benedictis LD et al (2011) Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-κB/COX‑2 signaling in human aortic endothelial cells. Am J Physiol Endocrinol Metab 301(6):E1143–E1154
Huang DG, Zhao QL, Liu HF et al (2016) PPAR‑α agonist WY-14643 inhibits LPS-induced inflammation in synovial fibroblasts via NF-kB pathway. J Mol Neurosci 59(4):544–553
Acknowledgements
The experiments were performed at Biological Medical Research Center of Zhongshan Hospital, Fudan University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
L.-j. Kong, Y.-n. Wang, Z. Wang and Q.-Z. Lv declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
Rights and permissions
About this article
Cite this article
Kong, Lj., Wang, Yn., Wang, Z. et al. NOD2 induces VCAM-1 and ET-1 gene expression via NF-κB in human umbilical vein endothelial cells with muramyl dipeptide stimulation. Herz 46 (Suppl 2), 265–271 (2021). https://doi.org/10.1007/s00059-020-04996-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-020-04996-y
Keywords
- Nucleotide-Binding Oligomerization Domain-Containing Protein 2 (NOD2)
- Vascular cell adhesion molecule‑1
- Endothelin‑1
- Endothelial dysfunction
- Cardiovascular diseases