Abstract
Background
We developed an atherosclerotic rabbit model and tested pioglitazone as a drug intervention for early vascular calcification. Positron emission tomography/computed tomography (PET/CT) was used to evaluate inflammation and therapeutic effects.
Methods
We randomly divided 20 male New Zealand white rabbits into a pioglitazone-treated group (n = 10) and a control group (n = 10). Atherosclerosis was induced via a high-cholesterol diet and endothelial denudation. The animals were maintained on a hyperlipidemic diet for 16 weeks after surgery, and the treatment group received pioglitazone daily. Serum samples were obtained at 8 and 18 weeks postoperatively to assess high-sensitivity C‑reactive protein (hs-CRP) and matrix metalloproteinase-9 (MMP-9) concentrations. Sixteen rabbits underwent a mid-stage PET/CT scan at week 8, and 11 rabbits underwent an end-stage PET/CT scan at week 18. PET/CT parameters, including the mean standardized uptake value (SUVmean) and maximum standardized uptake value (SUVmax), were measured and documented.
Results
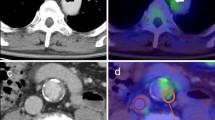
There were significantly lower hs-CRP and MMP-9 levels in the pioglitazone group at week 18 (p < 0.01). At the end of the 8th week, no significant between-group differences in SUVmean and SUVmax were observed. From week 8 to week 18, the SUVmean and SUVmax decreased in the pioglitazone group but the SUVmean increased in the control group, with significant between-group differences at the end of the 18th week (p < 0.01). Histopathological examination of aortas in the control and pioglitazone groups revealed significantly smaller plaque area, macrophage density, and tissue calcification area in the latter group.
Conclusion
Pioglitazone affects early vascular microcalcification, and pioglitazone-induced changes can be assessed using 18F-FDG-PET/CT.
Zusammenfassung
Hintergrund
Die Autoren entwickelten ein Kaninchenmodell der Arteriosklerose und testeten Pioglitazon als medikamentöse Intervention bei Gefäßverkalkung im Frühstadium. Zur Beurteilung entzündlicher Veränderungen und therapeutischer Auswirkungen wurde die Positronenemissionstomographie-Computertomographie (PET-CT) eingesetzt.
Methoden
Es wurden 20 männliche weiße Neuseelandkaninchen in eine pioglitazonbehandelte Gruppe (n = 10) und eine Kontrollgruppe (n = 10) eingeteilt. Arteriosklerose wurde durch Gabe von Nahrung mit hohem Cholesteringehalt und endotheliale Denudierung erzeugt. Die Tiere wurden 16 Wochen lang postoperativ bei Gabe hyperlidpidämischer Nahrung gehalten, dabei erhielt die Behandlungsgruppe täglich Pioglitazon. Serumproben wurden sowohl 8 als auch 18 Wochen nach Operation entnommen, um die Konzentration an hochsensitivem C‑reaktivem Protein (hs-CRP) und Matrixmetalloproteinase-9 (MMP-9) zu ermitteln. Bei 16 Kaninchen wurden nach der Hälfte der Untersuchungsdauer in Woche 8 eine PET-CT durchgeführt, bei 11 Kaninchen erfolgte dies zum Ende der Untersuchungsdauer in Woche 18. Dabei wurden PET-CT-Parameter einschließlich des mittleren standardisierten Aufnahmewerts („mean standardized uptake value“, SUVmean) und des maximalen standardisierten Aufnahmewerts („maximum standardized uptake value“, SUVmax) gemessen und dokumentiert.
Ergebnisse
In der Pioglitazongruppe bestanden in Woche 18 signifikant niedrigere hs-CRP- und MMP-9-Werte (p < 0,01) als bei den Kontrollen. Am Ende der 8. Woche fanden sich keine signifikanten Unterschiede bei SUVmean und SUVmax. Von Woche 8 bis Woche 18 nahmen der SUVmean und der SUVmax in der Pioglitazongruppe ab, in der Kontrollgruppe stieg der SUVmean jedoch an, dabei gab es signifikante Unterschiede zwischen den Gruppen am Ende der 18. Woche (p < 0,01). Die histopathologische Untersuchung der Aorten in der Kontroll- und der Pioglitazongruppe zeigte bei Letzterer signifikant kleinere Ausmaße von Plaques, Makrophagendichte und Bereichen mit Gewebeverkalkung.
Schlussfolgerung
Pioglitazon beeinflusst frühe vaskuläre Mikroverkalkungen, und durch Pioglitazon induzierte Veränderungen können mit der 18F-Fluordeoxyglukose(18F-FDG)-PET-CT nachgewiesen werden.



Similar content being viewed by others
References
Mizuno Y, Jacob RF, Mason RP (2011) Inflammation and the development of atherosclerosis [J]. J Atheroscler Thromb 18(5):351–358
Towler DA, Demer LL (2011) Thematic series on the pathobiology of vascular calcification: an introduction. Circ Res 108:1378–1380
Vengrenyuk Y, Carlier S, Xanthos S et al (2006) A hypothesis for vulnerable plaque rupture due to stressinduced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci Usa 103:14678–14683
Parulkar AA, Pendergrass ML, Granda-Ayala R et al (2001) Nonhypoglycemic effects of thiazolidinediones [J]. Ann Intern Med 134(1):61–71
Rangwala SM, Lazar MA (2004) Peroxisome proliferator-activated receptor gamma in diabetes and metabolism [J]. Trends Pharmacol Sci 25(6):331–336
Cariou B, Charbonnel B, Staels B (2012) Thiazolidinediones and PPARgamma agonists: Time for a reassessment [J]. Trends Endocrinol Metab 23(5):205–215
Deeg MA, Tan MH (2008) Pioglitazone versus Rosiglitazone: Effects on lipids, lipoproteins, and apolipoproteins in head-to-head randomized clinical studies [J]. PPAR Res:520465. https://doi.org/10.1007/s00059-017-4620-z
Krishnaswami A, Ravi-Kumar S, Lewis JM (2010) Thiazolidinediones: a 2010 perspective [J]. Perm J 14(3):64–72
Lincoff AM, Wolski K, Nicholls SJ et al (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: A meta-analysis of randomized trials. JAMA 298:1180–1188
Hutcheson JD, Maldonado N, Aikawa E (2014) Small entities with large impact: microcalcifications and atherosclerotic plaque vulnerability. Curr Opin Lipidol 25(5):327–332
Mohler ER 3rd (2004) Mechanisms of aortic valve calcification. Am J Cardiol 94:1396–1402
Rajamannan NM, Bonow RO, Rahimtoola SH (2007) Calcific aortic stenosis: an update. Nat Clin Pract Cardiovasc Med 4(5):254–262
Otto CM (2008) Calcific aortic stenosis—time to look more closely at the valve. N Engl J Med 359(13):1395–1398
Beheshti M, Saboury B, Mehta NN et al (2011) Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography—a novel concept. Hell J Nucl Med 14:114–120
Folco EJ, Sheikine Y, Rocha VZ et al (2011) Hypoxia but not inflammation augments glucose uptake in human macrophages: Implications for imaging atherosclerosis with 18fluorine-labeled 2‑deoxy-dglucose positron emission tomography. J Am Coll Cardiol 58:603–614
Hyafil F, Messika-Zeitoun D, Burg S et al (2012) Detection of 18fluoride sodium accumulation by positron emission tomography in calcified stenotic aortic valves. Am J Cardiol 109:1194–1196
Aikawa E, Otto CM (2012) Look more closely at the valve: imaging calcific aortic valve disease. Circulation 125:9–11
Dweck MR, Jenkins WS, Vesey AT et al (2014) 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 7(2):371–378
Dweck MR, Jones C, Joshi NV et al (2012) Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 125:76–86
Aronow WS, Ahn C, Kronzon I, Goldman ME (2001) Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol 88(6):693–695
Bellamy MF, Pellikka PA, Klarich KW et al (2002) Association of cholesterol levels, hydroxymethylglutaryl coenzyme—a reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol 40(10):1723–1730
Novaro GM, Tiong IY, Pearce GL et al (2001) Effect of hydroxymethylglutaryl coenzyme a reductase inhibitors on the progression of calcific aortic stenosis. Circulation 104:2205–2209
Cowell SJ, Newby DE, Prescott RJ et al (2005) A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352:2389–2397
Rossebo AB, Pedersen TR, Boman K et al (2008) Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 359:1343–1356
Ricote M, Li AC, Willson TM et al (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79–82
Moraes LA, Piqueras L, Bishop-Bailey D (2006) Peroxisome proliferator-activated receptors and inflammation. Pharmacol Ther 110:371–385
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Graham DJ, Ouellet-Hellstrom R, Macurdy TE et al (2010) Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone[J. JAMA 304(4):411–418
Wang N, Yin R, Liu Y et al (2011) Role of peroxisome proliferator-activated receptor-gamma in atherosclerosis: an update. Circ J 75(3):528–535
Vucic E, Dickson SD, Calcagno C et al (2011) Pioglitazone modulates vascular inflammation in atherosclerotic rabbits noninvasive assessment with FDG-PET-CT and dynamic contrast-enhanced MR imaging. JACC Cardiovasc Imaging 4(10):1100–1109
Stewart BF, Siscovick D, Lind BK et al (1997) Clinical factors associated with calcific aortic valve disease.Cardiovascular Health Study. J Am Coll Cardiol 29:630–634
Funding
This study was supported by the “Twelve Five” National Key Technology R & D Program of China (Grant No. 2011BAI11B05) (http://www.most.gov.cn/eng/programmes1/200610/t20061009_36224.htm), the Beijing Natural Science Foundation of China (Grant No. 7132078) (http://www.bjkw.gov.cn/n244495/index.html) and the National Natural Science Foundation of China (Grant No. 81370437) (https://isisn.nsfc.gov.cn/egrantindex/funcindex/prjsearch-list). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Xu, M. Nie, J. Li, Z. Xu, M. Zhang, Y. Yan, T. Feng, X. Zhao, and Q. Zhao declare that they have no competing interests.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Xu, J., Nie, M., Li, J. et al. Effect of pioglitazone on inflammation and calcification in atherosclerotic rabbits. Herz 43, 733–740 (2018). https://doi.org/10.1007/s00059-017-4620-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4620-z
Keywords
- Coronary artery disease
- Vascular calcification
- Atherosclerotic plaque
- Hypoglycemic agents
- Positron emission tomography-computed tomography