Abstract
Background
It is not known whether older patients with acute heart failure (HF) receiving tolvaptan have decreased mortality rates and a better long-term prognosis than patients who receive furosemide. We conducted a systematic review of randomized controlled trials (RCTs) to address this issue.
Methods
The Medline, Embase, and Cochrane Library databases were searched for English-language RCTs published before September 2016 comparing tolvaptan with furosemide treatment in older patients (>65 years old) after acute HF. The primary outcomes assessed were 6‑month all-cause mortality and worsening renal function (WRF); the secondary outcomes were electrolyte disorders, hospital readmissions, and adverse events.
Results
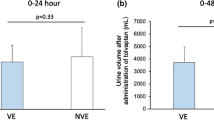
Out of 669 citations, six RCTs met the inclusion criteria for this meta-analysis. There was a significant decrease in WRF (relative risk [RR] = 0.67, 95% confidence interval [CI] = 0.52–0.86, p = 0.002) and in the hospitalization period (mean difference [MD] = −1.86, 95% CI = −3.70–−0.02, p = 0.05), as well as a significant increase in urine volume within 3 days of tolvaptan administration (MD = 1.59, 95% CI = 1.41–1.76, p < 0.00001). There were significant differences in creatinine levels between subgroups (MD = 0.33, 95% CI = 0.14–0.52, p = 0.0006). However, for the outcome of 6‑month all-cause mortality (RR = 0.56, 95% CI = 0.29–1.06, p = 0.07), there was no significant difference among all subgroups. There were significant differences in serum sodium concentration (MD = 0.68, 95% CI = 0.02–1.34, p = 0.04) but no significant changes in systolic blood pressure (MD = 3.57, 95% CI = −2.33–9.47, p = 0.24) between groups.
Conclusion
In older patients, tolvaptan relieves WRF, reduces the hospitalization period, and increases urine volume without significant effects on blood pressure. However, surprisingly, the use of tolvaptan did not influence 6‑month all-cause mortality.
Zusammenfassung
Hintergrund
Es ist nicht bekannt, ob ältere Patienten mit akuter Herzinsuffizienz unter Gabe von Tolvaptan eine geringere Mortalitätsrate und eine bessere Langzeitprognose als Patienten unter Gabe von Furosemid aufweisen. Die Autoren führten eine systematische Auswertung randomisierter kontrollierter Studien („randomized controlled trials“, RCT) durch, um die Frage zu klären.
Methoden
Die Datenbanken Medline, Embase und der Cochrane Library wurden nach englischsprachigen RCT durchsucht, die vor September 2016 publiziert worden waren und in denen die Tolvaptan- mit der Furosemidbehandlung bei älteren Patienten (>65 Jahre) nach akuter Herzinsuffizienz verglichen wurde. Primäre Endpunkte waren die 6‑Monats-Gesamtmortalität und eine sich verschlechternde Nierenfunktion („worsening renal function“, WRF); sekundäre Endpunkte waren Elektrolytstörungen, Wiederaufnahme ins Krankenhaus und unerwünschte Wirkungen.
Ergebnisse
Von 669 zitierten Arbeiten erfüllten 6 RCT die Einschlusskriterien dieser Metaanalyse. Es bestand eine signifikante Abnahme der WRF (relatives Risiko, RR: 0,67; 95%-Konfidenzintervall, 95%-KI: 0,52–0,86; p = 0,002) und der Verweildauer (Mittelwertdifferenz, MD: −1,86; 95%-KI: −3,70 bis −0,02; p = 0,05) sowie ein signifikanter Anstieg des Urinvolumens innerhalb von 3 Tagen nach Tolvaptangabe (MD: 1,59; 95%-KI: 1,41–1,76; p < 0,00001). Zwischen den Untergruppen zeigten sich signifikante Unterschiede beim Kreatininwert (MD: 0,33; 95%-KI: 0,14–0,52; p = 0,0006). Jedoch fand sich kein signifikanter Unterschied zwischen allen Untergruppen für die 6‑Monats-Gesamtmortalität (RR: 0,56; 95%-KI: 0,29–1,06; p = 0,07). Signifikante Unterschiede zwischen den Gruppen bestanden bei der Natriumkonzentration im Serum (MD: 0,68; 95%-KI: 0,02–1,34; p = 0,04), aber nicht beim systolischen Blutdruck (MD: 3,57; 95%-KI: −2,33 bis 9,47; p = 0,24).
Schlussfolgerung
Bei älteren Patienten führt Tolvaptan zur Verminderung der WRF, zur Senkung der Hospitalisierungsdauer und zur Steigerung des Urinvolumens ohne signifikante Wirkung auf den Blutdruck. Jedoch beeinflusste überraschenderweise die Gabe von Tolvaptan die Gesamtmortalität nach 6 Monaten nicht.





Similar content being viewed by others
References
Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D et al (2013) Clinical course of patients with Hyponatremiaand decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 19(6):390–397
Imamura T, Kinugawa K (2016) Urine aquaporin-2: a promising marker of response to the arginine vasopressin type-2 antagonist, tolvaptan in patients with congestive heart failure. Int J Mol Sci 17(1):E105
Zulkifli AH, Suridanda DS (2016) Tolvaptan: a novel diuretic in heart failure management. J Tehran Heart Cent 11(1):1–5
Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T (2007) Tolvaptan, an orally active vasopressin V(2)-receptor antagonist – pharmacology and clinical trials. Cardiovasc Drug Rev 25(1):1–13
Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y et al (2013) Efficacy and safety of a 14-day administration of tolvaptan in the treatment ofpatients with ascites in hepatic oedema. J Int Med Res 41(3):835–847
Thajudeen B, Salahudeen AK (2016) Role of tolvaptan in the management of hyponatremia in patients with lung and other cancers: current data and future perspectives. Cancer Manag Res 8:105–114
Kawaratani H, Fukui H, Yoshiji H (2016) Treatment for cirrhotic ascites. Hepatol Res 47(2):166. doi:10.1111/hepr.12769
Guler S, Cimen S, Hurton S, Molinari M (2015) Diagnosis and treatment modalities of symptomatic polycystic kidney disease. In: Li X (ed) Polycystic kidney disease. Codon Publications, Brisbane, pp 1–10
Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A et al (2016) Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant 31(3):337–348
Kimura K, Momose T, Hasegawa T, Morita T, Misawa T, Motoki H et al (2016) Early administration of tolvaptan preserves renal function in elderly patients with acutedecompensated heart failure. J Cardiol 67(5):399–405
Kinoshita M, Okayama H, Kawamura G, Shigematsu T, Takahashi T, Kawada Y et al (2016) Favorable effect of early administration of tolvaptanin elderlypatients with repeat hospitalizations for acute decompensated heart failure. J Am Coll Cardiol 67(13, Supplement):1426
Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y et al (2016) Clinical effectiveness of tolvaptan in patientswith acute heartfailure and renal dysfunction. J Card Fail 22(6):423–432
Matsuzaki M, Hori M, Izumi T, Fukunami M (2011) Efficacy and safety of tolvaptan in heart failure patientswith volume overload despite the standard treatmentwith conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther 25(S1):S33–S45. doi:10.1007/s10557-011-6304-x
Uemura Y, Shibata R, Takemoto K, Uchikawa T, Koyasu M, Ishikawa S et al (2016) Clinical benefit of tolvaptan in patients with acute decompensatedheart failure and chronic kidney disease. Heart Vessel 31(10):1643–1649
Matsue Y, Suzuki M, Seya M, Iwatsuka R, Mizukami A et al (2013) Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure in high-risk population. J Cardiol 61(2):169–174
Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M et al (2003) Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 107:2690–2696
Goldsmith SR (2016) A new approach to treatment of acute heart failure. J Cardiol 67(5):395–398
Foebel AD, Heckman GA, Ji K, Dubin JA, Turpie ID, Hussack P et al (2013) Heart failure – related mortality and hospitalization inthe year following admission toa long-term carefacility:the geriatric outcomes and longitudinal decline in heart failure (GOLD-HF) study. J Card Fail 19(7):468–477
Xiong B, Huang Y, Tan J, Yao Y, Wang C, Qian J et al (2015) The short-term and long-term effects of tolvaptan in patientswith heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev 20(6):633–642
Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C et al (2006) Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium andpotassium excretion in human heart failure. Am J Physiol Renal Physiol 290(2):F273–F278
Abo-Salem E, Sherif K, Dunlap S, Prabhakar S (2014) Potential aetiologies and prognostic implications of worsening renal function in acute decompensated heart failure. Acta Cardiol 69(6):657–663
Gheorghiade M, Pang PS, Ambrosy AP, Lan G, Schmidt P, Filippatos G et al (2012) A comprehensive, longitudinal description of the in-hospitaland post-discharge clinical, laboratory, and neurohormonalcourse of patients with heart failure who die or are re-hospitalized within 90 days: analysis from the EVEREST trial. Heart Fail Rev 17(3):485–509
Imamura T, Kinugawa K, Minatsuki S, Muraoka H, Kato N, Inaba T et al (2013) Urine osmolality estimated using urine urea nitrogen, sodium and creatinine can effectively predict response to tolvaptan in decompensated heart failure patients. Circ J 77:1208–1213
Shanmugam E, Doss CR, George M, Jena A, Rajaram M, Ramaraj B et al (2016) Effect of tolvaptan on acute heart failure with hyponatremia – a randomized, double blind, controlledclinical trial. Indian Heart J 68:S15–S21. doi:10.1016/j.ihj.2015.07.006
Imamura T, Kinugawa K, Nitta D, Komuro I (2016) Tolvaptan reduces long-term total medical expenses and length of stay in aquaporin-defined responders. Int Heart J 57(5):593–599
Eguchi A, Iwasaku T, Okuhara Y, Naito Y, Mano T, Masuyama T et al (2016) Long-term administration of tolvaptan increases myocardial remodelingand mortality via exacerbation of congestion in mice heart failure modelafter myocardial infarction. Int J Cardiol 221:302–309
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81170133 to J. Yang; Grant No. 81200088,81470387 to J. Yang), the Natural Science Foundation of Yichang City, China (Grant No.A12301-01), and Hubei Province’s Outstanding Medical Academic Leader Program, China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
W.-l. Huang, Y. Yang, J. Yang, J. Yang, H.-B. Wang, X.-l. Xiong, and Y.-f. Zhang declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Wei-ling Huang and Ying Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, W., Yang, Y., Yang, J. et al. Use of tolvaptan vs. furosemide in older patients with heart failure. Herz 43, 338–345 (2018). https://doi.org/10.1007/s00059-017-4563-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-017-4563-4