Abstract
Background
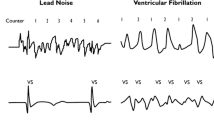
A new implantable cardiac monitor (BioMonitor, Biotronik) with a continuous remote monitoring option was prospectively implanted in patients with suspected arrhythmias or for therapy control after atrial fibrillation (AF) ablation. A three-lead ECG detection was intended to make the implantation more independent of the implantation site and the electrical heart axis. Because noise is a frequent problem in implantable cardiac monitors, an active noise detection algorithm was implemented. The aim of the trial was to evaluate the clinical performance of the device.
Methods
The device performance was evaluated in a prospective nonrandomized multicenter study with a follow-up of 12 months. Study endpoints were device-related serious adverse events at 3 months, appropriate QRS detection in direct comparison with synchronized Holter ECG recordings, sensitivity and positive predictive value of arrhythmia detection in comparison with Holter ECG and independent of it, and noise burden during the entire follow-up period.
Results
The implantation was successful in all 152 patients. Two device-related serious adverse events (pocket infections) occurred by 3 months. The mean QRS amplitude of 0.3 ± 0.2 mV at implantation remained stable over 12 months. QRS sensing performance indicated little over- and undersensing in most patients. More than 80 % of the patients had more than 22 h of noise-free monitoring per day.
Conclusion
BioMonitor effectively detects patients with bradycardia, tachycardia, AF, or asystole. Active noise detection seems to reduce the transmission of meaningless data without diminishing the positive predictive value of the device.
Zusammenfassung
Zielsetzung
Ein neu entwickelter implantierbarer Herzmonitor (BioMonitor, Biotronik) mit der Option der kontinuierlichen Fernüberwachung wurde prospektiv bei Patienten mit vermuteten Arrhythmien oder zur Therapiekontrolle nach Vorhofflimmerablation implantiert. Das im Gerät integrierte 3‑Kanal-EKG sollte die Implantation flexibler bezüglich des Implantationsorts und der elektrischen Herzachse machen. Da Artefaktsensing ein häufiges Problem darstellt, wurde in das Gerät ein Algorithmus zur aktiven Artefakterkennung integriert. Ziel der Studie war es, die Funktionalität im klinischen Alltag zu prüfen.
Methoden
Diese multizentrische Studie erfolgte prospektiv nichtrandomisiert mit einem Follow-up von 12 Monaten. Studienendpunkte waren geräteassoziierte schwerwiegende unerwünschte Ereignisse („serious adverse events“, SAE) 3 Monate nach Implantation, korrekte QRS-Detektion im Vergleich zu synchronisierten Langzeit-EKGs, Sensitivität und positiv-prädiktiver Wert der Arrhythmiedetektion im Vergleich zu synchronisierten Langzeit-EKGs und unabhängig davon sowie die Artefaktlast während der gesamten Nachbeobachtungszeit.
Ergebnisse
Die Implantation verlief bei allen 152 Patienten erfolgreich. Es traten 2 geräteassoziierte SAE auf (Tascheninfektionen). Die mittlere QRS-Amplitude von 0,3 ± 0,2 mV bei Implantation blieb über 12 Monate stabil. Bezüglich des QRS-Sensings zeigte sich bei den meisten Patienten ein geringes Over- oder Undersensing. Über 80 % der Patienten wiesen eine artefaktfreie Überwachung von mindestens 22 h pro Tag auf.
Schlussfolgerungen
Der BioMonitor detektiert Bradykardien, Tachykardien inklusive Vorhofflimmern und Asystolien effektiv. Die aktive Artefakterkennung reduziert dabei die Übertragung nutzloser Daten, ohne den positiv-prädiktiven Wert der Arrhythmieerkennung zu verringern.





Similar content being viewed by others
References
Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F et al (2011) Catheter ablation for atrial fibrillation: are results maintained at 5years of follow-up? J Am Coll Cardiol 57:160–166
Brignole M, Vardas P, Hoffman E, Huikuri H, Moya A, Ricci R et al (2009) Indications for the use of diagnostic implantable and external ECG loop recorders. Europace 11:671–687
Grubb BP, Welch M, Kanjwal K, Karabin B, Kanjwal Y (2010) An anatomic-based approach for the placement of implantable loop recorders. Pacing Clin Electrophysiol 33:1149–1152
Podd SJ, Sugihara C, Furniss SS, Sulke N (2015) Are implantable cardiac monitors the ‘gold standard’ for atrial fibrillation detection? A prospective randomized trial comparing atrial fibrillation monitoring using implantable cardiac monitors and DDDRP permanent pacemakers in post atrial fibrillation ablation patients. Europace 18(7):1000–1005. doi:10.1093/europace/euv367
Acknowledgements
The authors thank Frank Kleinjung for biostatistical support, Jürgen Schrader and Dejan Danilovic for editorial assistance, and last but not least Gundala Hermann for the project management of the whole study.
Funding
This work was supported by Biotronik SE & Co. KG, Berlin, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J. Lauschke received speaker honoraria and consulting fees from Biotronik. M. Busch received speaker honoraria, consulting fees, and research grants from Biotronik. H. Nägele received research grants and speaker honoraria from Biotronik. D. Bänsch received speaker honoraria, consulting fees, and research grants from Biotronik. R. Schneider received consulting fees from Biotronik. W. Haverkamp, A. Bulava, D. Andresen, C. Israel, and G. Hindricks declare that they have no competing interest. Biotronik was involved in the study design, data collection and the analysis of the data.
Rights and permissions
About this article
Cite this article
Lauschke, J., Busch, M., Haverkamp, W. et al. New implantable cardiac monitor with three-lead ECG and active noise detection. Herz 42, 585–592 (2017). https://doi.org/10.1007/s00059-016-4492-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-016-4492-7