Abstract
Objectives
The aim of this study was to investigate whether renal sympathetic denervation (RSD) is more effective on myocardial hypertrophy than the angiotensin-converting enzyme inhibitor (ACEI) perindopril in spontaneously hypertensive rats (SHRs).
Methods
After bilateral renal denervation blood pressure (BP) was measured every 10 days. On day 50 the heart was (histo)pathologically examined. The ventricular weight to body weight ratios (VW/BW), myocardial diameter and collagen volume fraction (CVF) were calculated, and cardiac hypertrophy marker genes were analyzed by RT-PCR.
Results
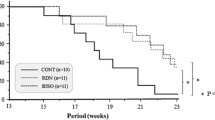
At the baseline evaluation all groups had comparable BP. After treatment the BP of the RSD group was significantly reduced (p < 0.05). The BP of the RSD group was lower than that of the perindopril group on days 10, 20 and 30th (p < 0.05) but on day 50 systolic BP of the RSD group was significantly higher (p < 0.05) whereas there were no significant differences in diastolic BP. The VW/BW decreased in the RSD group, whereas the value did not change significantly in the perindopril group. The myocardial diameter of the left ventricular cardiomyocytes was also significantly lower in the RSD group and stayed the same in the perindopril group. Collagen volume fraction (CVF) in the RSD group was significantly lower than in the perindopril group (p < 0.05). Significant changes in the expression levels of NPPA, MYH7, and MYH6 (P < 0.05) were observed in the RD-SHR groups (p < 0.05). There was a significant difference in the expression level of MYH6 (p < 0.05) between the RSD group and the perindopril group but the expression levels of NPPA and MYH7 were not significantly different.
Conclusion
In this study, RSD had a significant antihypertensive effect and inhibited hypertensive-induced cardiac hypertrophy in SHRs and showed advantages compared with ACEI in decreasing BP in the early stage and in inhibiting myocardial fibrosis.
Zusammenfassung
Ziel
Ziel der Studie war zu untersuchen, ob bei Ratten mit spontan aufgetretener Hypertonie (SHR) und Myokardhypertrophie die renale Sympathikusdenervation (RSD) wirksamer als der Angiotensin-converting-Enzym-Inhibitor (ACEI) Perindopril.
Methoden
Nach beidseitiger renaler Denervation wurde der Blutdruck (RR) alle 10 Tage gemessen. Am Tag 50 wurde das Herz histopathologisch untersucht. Das Verhältnis von Ventrikelgewicht zu Körpergewicht (VW/BW), Myokarddurchmesser und Kollagenvolumenfraktion (CVF) wurden ermittelt, Markergene für kardiale Hypertrophie wurden per Echtzeitpolymerasekettenreaktion (RT-PCR) analysiert.
Ergebnisse
Bei der Eingangsuntersuchung wiesen alle Gruppen einen vergleichbaren RR auf. Nach Therapie war der RR der RSD-Gruppe signifikant vermindert (p < 0,05). Der RR in der RSD-Gruppe war an Tag 10, 20 und 30 niedriger als in der Perindopril-Gruppe (p < 0,05), aber an Tag 50 war der systolische RR der RSD-Gruppe signifikant höher (p < 0,05), während es keine signifikanten Unterschiede beim diastolischen RR gab. Der VW/BW-Wert nahm in der RSD-Gruppe zwar ab, doch er änderte sich in der Perindopril-Gruppe nicht signifikant. Der anhand der linksventrikulären Kardiomyozyten bestimmte Myokarddurchmesser war in der RSD-Gruppe ebenfalls signifikant geringer und blieb in der Perindopril-Gruppe gleich. Die CVF war in der RSD-Gruppe signifikant niedriger als in der Perindopril-Gruppe (p < 0,05). Signifikante Veränderungen des Expressionsgrads von NPPA, MYH7 und MYH6 (p < 0,05) wurden in der RSD-SHR-Gruppe festgestellt (p < 0,05). Es bestand ein signifikanter Unterschied beim Expressionsgrad von MYH6 (p < 0,05) zwischen der RSD-Gruppe und der Perindopril-Gruppe, nicht jedoch beim Expressiongrad von NPPA und MYH7.
Schlussfolgerung
In dieser Studie hatte die RSD eine signifikante antihypertensive Wirkung, sie hemmte die hypertonieinduzierte kardiale Hypertrophie bei SHR und wies Vorteile im Vergleich zum ACEI bei der RR-Senkung im Frühstadium sowie bei der Hemmung der Myokardfibrose auf.






Similar content being viewed by others
References
Diamond JA, Phillips RA (2005) Hypertensive heart disease. Hypertens Res 28:191–202
Agabiti-Rosei E, Muiesan ML (2002) Left ventricular hypertrophy and heart failure in women. J Hypertens Suppl 20:S34–S38
Ruilope LM, Schmieder RE (2008) Left ventricular hypertrophy and clinical outcomes in hypertensive patients. Am J Hypertens 21:500–508
Lindholm LH, Carlberg B, Samuelsson O (2005) Should beta blockers remain first choice in the treatment of primary hypertension? A meta-analysis. Lancet 366:1545–1553
Williams B, Lacy PS, Thom SM et al (2006) Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113:1213–1225
Yang CM, Kandaswamy V, Young D, Sen S (1997) Changes in collagen phenotypes during progression and regression of cardiac hypertrophy. Cardiovasc Res 36:236–245
Brilla CG, Janicki JS, Weber KT (1991) Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res 69:107–115
Brooks WW, Bing OH, Robinson KG et al (1997) Effect of angiotensin-converting enzyme inhibition on myocardial fibrosis and function in hypertrophied and failing myocardium from the spontaneously hypertensive rat. Circulation 96:4002–4010
Brilla CG, Pick R, Tan LB et al (1990) Remodeling of the rat right and left ventricles in experimental hypertension. Circ Res 67:1355–1364
Brilla CG, Matsubara LS, Weber KT (1993) Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am J Cardiol 71:12A–16A
Mancia G, Grassi G, Giannattasio C, Seravalle G (1999) Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 34:724–728
Pimenta E, Oparil S (2012) Renal sympathetic denervation for treatment of hypertension. Curr Treat Options Cardiovasc Med in press
Brandt MC, Mahfoud F, Reda S et al (2012) Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol 59:901–909
Bombelli M, Facchetti R, Carugo S et al (2009) Left ventricular hypertrophy increases cardiovascular risk independently of in-office and out-of-office blood pressure values. J Hypertens 27:2458–2464
Franklin SS, Wachtell K, Papademetriou V et al (2005) Cardiovascular morbidity and mortality in hypertensive patients with lower versus higher risk: a life substudy. Hypertension 46:492–499
Asai K, Yang GP, Geng YJ et al (1999) Beta-adrenergic receptor blockade arrests myocyte damage and preserves cardiac function in the transgenic G (salpha) mouse. J Clin Invest 104:551–558
Krum H, Schlaich M, Whitbourn R et al (2009) Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373:1275–1281
Perlini S, Palladini G, Ferrero I et al (2005) Sympathectomy or doxazosin, but not propranolol, blunt myocardial interstitial fibrosis in pressure-overload hypertrophy. Hypertension 46:1213–1218
Levick SP, Murray DB, Janicki JS, Brower GL (2010) Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension 55:270–276
Schlaich MP, Sobotka PA, Krum H et al (2009) Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361:932–934
DiBona GF (2004) The sympathetic nervous system and hypertension: recent developments. Hypertension 43:147–150
Wyss JM, Oparil S, Sripairojthikoon W (1992) Neuronal control of the kidney: contribution to hypertension. Can J Physiol Pharmacol 70:759–770
Brilla CG, Zhou G, Matsubara L, Weber KT (1994) Collagen metabolism in cultured adult rat cardiac fibroblasts: response to angiotensin II and aldosterone. J Mol Cell Cardiol 26:809–820
Villarreal FJ, Kim NN, Ungab GD et al (1993) Identification of functional angiotensin ii receptors on rat cardiac fibroblasts. Circulation 88:2849–2861
Chevalier B, Heudes D, Heymes C et al (1995) Trandolapril decreases prevalence of ventricular ectopic activity in middle-aged SHR. Circulation 92:1947–1953
Dias LD, Casali KR, Leguisamo NM et al (2011) Renal denervation in an animal model of diabetes and hypertension: impact on the autonomic nervous system and nephropathy. Cardiovasc Diabetol 10:33
Compliance with ethical guidelines
Conflict of interest. Xueyan Ding, Xudong Xu, Yan Yan, Xiaowei Song, Suxuan Liu, Guokun Wang, Dingfeng Su, Qing Jing, and Yongwen Qin state that there are no conflicts of interest. All national guidelines on the care and use of laboratory animals have been followed and the necessary approval was obtained from the relevant authorities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xueyan Ding and Xudong Xu contributed equally to this study..
Rights and permissions
About this article
Cite this article
Ding, X., Xu, X., Yan, Y. et al. Effects of renal sympathetic denervation and angiotensin-converting enzyme inhibitor on left ventricular hypertrophy. Herz 40, 695–701 (2015). https://doi.org/10.1007/s00059-014-4110-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-014-4110-5
Keywords
- Renal sympathetic denervation
- Angiotensin-converting enzyme inhibitor
- Myocardial hypertrophy
- Spontaneously hypertensive rats
- Rats, inbred strain