Abstract
Purpose
To investigate in vitro the impact of fibroblast growth factor 1 (FGF1) in comparison to ascorbic acid (AscA) on human periodontal ligament fibroblast (HPdLF) growth, their osteogenic differentiation, and modulation of their inflammatory reaction to mechanical stress.
Methods
The influence of different concentrations of FGF1 (12.5–200 ng/mL) on growth and proliferation of HPdLF cells was analyzed over 20 days by counting cell numbers and the percentage of Ki67-positive cells. Quantitative expression analysis of genes encoding the osteogenic markers alkaline phosphatase (ALPL), Runt-related transcription factor 2 (RUNX2), osteocalcin (OCN), and osteopontin (OSP), as well as the fibroblast markers vimentin (VIM) and fibroblast-specific protein 1 (FSP1), was performed after 2 and 20 days of cultivation. Metabolic activity was determined by MTT assay. For comparison with AscA, 50 ng/mL FGF1 was used for stimulation for 2 and 20 days. Cell number, percentage of Ki67-positive cells, and expression of osteoblast- and fibroblast-specific genes were examined. Alkaline phosphatase activity was visualized by NBT/BCIP and calcium deposits were stained with alizarin red. Cytokine (IL‑6, IL‑8, COX2/PGE2) expression and secretion were analyzed by qPCR and ELISA in 6 h mechanically compressed HPdLF cultured for 2 days with FGF1 or ascorbic acid.
Results
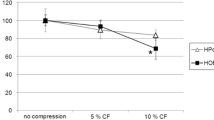
Higher concentrations of FGF1 promoted cell proliferation upon short-term stimulation, whereas prolonged treatment induced the expression of osteogenic markers even with low concentrations. AscA promotes cell growth more markedly than FGF1 in short-term cultures, whereas FGF1 induced osteogenic cell fate more strongly in long-term culture. Both factors induced an increased inflammatory response of HPdLF to mechanical compression.
Conclusion
Our data suggest that FGF1 promotes an osteogenic phenotype of HPdLF and limits inflammatory response to mechanical forces compared to AscA.
Zusammenfassung
Zielsetzung
In-vitro-Evaluation des Einflusses von Fibroblastenwachstumsfaktor 1 (FGF1) im Vergleich zu Ascorbinsäure (AscA) auf das Wachstum, das osteogene Differenzierungspotenzial und die inflammatorische Stressreaktion humaner Parodontalligamentfibroblasten (HPdLF).
Methoden
Der Einfluss von verschiedenen Konzentrationen von FGF1 (12,5–200 ng/ml) auf das Wachstum und die Proliferation von HPdLF-Zellen wurde über 20 Tage anhand von Zellzahlen und dem Anteil an Ki67-positiven Zellen analysiert. Die quantitative Expressionsanalyse von Genen, welche für die osteogenen Marker alkalische Phosphatase (ALPL), „runt-related transcription factor 2“ (RUNX2), Osteocalcin (OCN) und Osteopontin (OSP) sowie für die Fibroblastenmarker Vimentin (VIM) und „fibroblast-specific protein 1“ (FSP1) kodieren, wurde nach 2 und 20 Tagen Kultivierung durchgeführt. Die metabolische Aktivität wurde mittels MTT-Assay bestimmt. Für den Vergleich mit AscA wurde 50 ng/ml FGF1 zur Stimulation für 2 und 20 Tage verwendet. Die Zellzahl, der Anteil Ki67-positiver Zellen und die Expression von osteoblasten- und fibroblastenspezifischen Genen wurden untersucht. Die Aktivität der alkalischen Phosphatase wurde mittels NBT/BCIP visualisiert und Kalziumablagerungen mit Alizarin-Rot angefärbt. Die Expression und die Sezernierung von Zytokinen (IL‑6, IL‑8, COX2/PGE2) wurden mittels qPCR und ELISA in 6 h mechanisch komprimierten HPdLF analysiert, die 2 Tage mit FGF1 oder Ascorbinsäure kultiviert wurden.
Ergebnisse
Höhere Konzentrationen von FGF1 förderten die Zellproliferation bei Kurzzeitstimulation, während bei längerer Behandlung auch mit niedrigen Konzentrationen die Expression osteogener Marker induziert wurde. AscA förderte das Zellwachstum in Kurzzeitkulturen deutlicher als FGF1, wohingegen FGF1 den osteogenen Phänotyp bei Langzeitkultivierung stärker hervorrief. Beide Faktoren induzierten eine erhöhte inflammatorische Reaktion von HPdLF auf mechanische Kompression.
Schlussfolgerung
Unsere Daten deuten darauf hin, dass FGF1 den osteogenen Phänotyp von HPdLF bei Langzeitkultivierung fördert und die immunologische Stressreaktion auf mechanische Kräfte im Vergleich zu AscA mindert.




Similar content being viewed by others
References
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129:469.e1–469.32. https://doi.org/10.1016/j.ajodo.2005.10.007
Vansant L, Cadenas De Llano-Pérula M, Verdonck A, Willems G (2018) Expression of biological mediators during orthodontic tooth movement: a systematic review. Arch Oral Biol 95:170–186. https://doi.org/10.1016/j.archoralbio.2018.08.003
Reitan K (1967) Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod Dentofacial Orthop 53:721–745
Niklas A, Proff P, Gosau M, Römer P (2013) The role of hypoxia in orthodontic tooth movement. Int J Dent. https://doi.org/10.1155/2013/841840
Wolf M, Lossdörfer S, Küpper K, Jäger A (2014) Regulation of high mobility group box protein 1 expression following mechanical loading by orthodontic forces in vitro and in vivo. Eur J Orthod 36:624–631. https://doi.org/10.1093/ejo/cjt037
Wolf M, Lossdörfer S, Craveiro R et al (2013) Regulation of macrophage migration and activity by high-mobility group box 1 protein released from periodontal ligament cells during orthodontically induced periodontal repair: an in vitro and in vivo experimental study. J Orofac Orthop 74:420–434. https://doi.org/10.1007/s00056-013-0167-7
Henneman S, Von den Hoff Maltha JJ (2008) Mechanobiology of tooth movement. Eur J Orthod 30:299–306. https://doi.org/10.1093/ejo/cjn020
Kheralla Y, Götz W, Kawarizadeh A, Rath-Deschner B, Jäger A (2010) IGF‑I, IGF-IR and IRS1 expression as an early reaction of PDL cells to experimental tooth movement in the rat. Arch Oral Biol 55(3):215–222. https://doi.org/10.1016/j.archoralbio.2010.01.002
Sakai Y, Balam TA, Kuroda S et al (2009) CTGF and apoptosis in mouse osteocytes induced by tooth movement. J Dent Res 88:345–350. https://doi.org/10.1177/0022034509334649
Tsuge A, Noda K, Nakamura Y (2016) Early tissue reaction in the tension zone of PDL during orthodontic tooth movement. Arch Oral Biol 65:17–25. https://doi.org/10.1016/j.archoralbio.2016.01.007
Yamashiro T, Fukunaga T, Kobashi N et al (2001) Mechanical stimulation induces CTGF expression in rat osteocytes. J Dent Res 80:461–465. https://doi.org/10.1177/00220345010800021201
Alhashimi N, Frithiof L, Brudvik P, Bakhiet M (2000) Orthodontic movement induces high numbers of cells expressing IFN-gamma at mRNA and protein levels. J Interferon Cytokine Res 20:7–12. https://doi.org/10.1089/107999000312685
Alhashimi N, Frithiof L, Brudvik P, Bakhiet M (2001) Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop 119:307–312. https://doi.org/10.1067/mod.2001.110809
Alikhani M, Alyami B, Lee IS et al (2015) Saturation of the biological response to orthodontic forces and its effect on the rate of tooth movement. Orthod Craniofac Res 18(Suppl 1):8–17. https://doi.org/10.1111/ocr.12090
Andrade IJ, Silva TA, Silva GAB et al (2007) The role of tumor necrosis factor receptor type 1 in orthodontic tooth movement. J Dent Res 86:1089–1094. https://doi.org/10.1177/154405910708601113
Baba S, Kuroda N, Arai C et al (2011) Immunocompetent cells and cytokine expression in the rat periodontal ligament at the initial stage of orthodontic tooth movement. Arch Oral Biol 56:466–473. https://doi.org/10.1016/j.archoralbio.2010.11.010
Bletsa A, Berggreen E, Fristad I et al (2006) Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. J Physiol 573:225–236. https://doi.org/10.1113/jphysiol.2006.104711
Hazan-Molina H, Reznick AZ, Kaufman H, Aizenbud D (2015) Periodontal cytokines profile under orthodontic force and extracorporeal shock wave stimuli in a rat model. J Periodontal Res 50:389–396. https://doi.org/10.1111/jre.12218
Kohara H, Kitaura H, Yoshimatsu M et al (2012) Inhibitory effect of interferon‑γ on experimental tooth movement in mice. J Interf Cytokine Res 32:426–431. https://doi.org/10.1089/jir.2011.0124
Liang Y, Zhou Y, Jiang T et al (2011) Expression of LIF and LIFR in periodontal tissue during orthodontic tooth movement. Angle Orthod 81:600–608. https://doi.org/10.2319/102510-622.1
Lina ILA, da Silva JM, Rodrigues LFD et al (2017) Contribution of atypical chemokine receptor 2/ackr2 in bone remodeling. Bone 101:113–122. https://doi.org/10.1016/j.bone.2017.05.003
de Taddei SR, Andrade IJ, Queiroz-Junior CM et al (2012) Role of CCR2 in orthodontic tooth movement. Am J Orthod Dentofacial Orthop 141:153–160. https://doi.org/10.1016/j.ajodo.2011.07.019
Yoshimatsu M, Shibata Y, Kitaura H et al (2006) Experimental model of tooth movement by orthodontic force in mice and its application to tumor necrosis factor receptor-deficient mice. J Bone Miner Metab 24:20–27. https://doi.org/10.1007/s00774-005-0641-4
Janjic Rankovic M, Docheva D, Wichelhaus A, Baumert U (2020) Effect of static compressive force on in vitro cultured PDL fibroblasts: monitoring of viability and gene expression over 6 days. Clin Oral Investig 24:2497–2511. https://doi.org/10.1007/s00784-019-03113-6
Kanzaki H, Chiba M, Shimizu Y, Mitani H (2002) Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 17:210–220. https://doi.org/10.1359/jbmr.2002.17.2.210
Somerman MJ, Young MF, Foster RA et al (1990) Characteristics of human periodontal ligament cells in vitro. Arch Oral Biol 35:241–247. https://doi.org/10.1016/0003-9969(90)90062-f
Nojima N, Kobayashi M, Shionome M et al (1990) Fibroblastic cells derived from bovine periodontal ligaments have the phenotypes of osteoblasts. J Periodontal Res 25:179–185. https://doi.org/10.1111/j.1600-0765.1990.tb01041.x
Powers CJ, McLeskey SW, Wellstein A (2000) Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer 7:165–197
Sluzalska K, Slawski J, Sochacka M et al (2020) Intracellular partners of fibroblast growth factors 1 and 2—implications for functions. Cytokine Growth Factor Rev. https://doi.org/10.1016/j.cytogfr.2020.05.004
Lemmon MA, Schlessinger J (2010) Cell signaling by receptor tyrosine kinases. Cell 141:1117–1134. https://doi.org/10.1016/j.cell.2010.06.011
Eswarakumar VP, Lax I, Schlessinger J (2005) Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev 16:139–149. https://doi.org/10.1016/j.cytogfr.2005.01.001
Mansukhani A, Bellosta P, Sahni M, Basilico C (2000) Signaling by fibroblast growth factors (FGF) and fibroblast growth factor receptor 2 (FGFR2)-activating mutations blocks mineralization and induces apoptosis in osteoblasts. J Cell Biol 149:1297–1308. https://doi.org/10.1083/jcb.149.6.1297
Imamura T, Oka S, Tanahashi T, Okita Y (1994) Cell cycle-dependent nuclear localization of exogenously added fibroblast growth factor‑1 in BALB/c 3T3 and human vascular endothelial cells. Exp Cell Res 215:363–372. https://doi.org/10.1006/excr.1994.1353
Więdłocha A, Falnes PØ, Madshus IH et al (1994) Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell 76:1039–1051. https://doi.org/10.1016/0092-8674(94)90381-6
Olsnes S, Klingenberg O, Wiedłocha A (2003) Transport of exogenous growth factors and cytokines to the cytosol and to the nucleus. Physiol Rev 83:163–182. https://doi.org/10.1152/physrev.00021.2002
Małecki J, Wesche J, Skjerpen CS et al (2004) Translocation of FGF‑1 and FGF‑2 across vesicular membranes occurs during G1-phase by a common mechanism. Mol Biol Cell 15:801–814. https://doi.org/10.1091/mbc.e03-08-0589
Sørensen V, Wiedlocha A, Haugsten EM et al (2006) Different abilities of the four FGFRs to mediate FGF‑1 translocation are linked to differences in the receptor C‑terminal tail. J Cell Sci 119:4332–4341. https://doi.org/10.1242/jcs.03209
Sørensen V, Zhen Y, Zakrzewska M et al (2008) Phosphorylation of fibroblast growth factor (FGF) receptor 1 at Ser777 by p38 mitogen-activated protein kinase regulates translocation of exogenous FGF1 to the cytosol and nucleus. Mol Cell Biol 28:4129–4141. https://doi.org/10.1128/MCB.02117-07
Cam Y, Neumann MR, Oliver L et al (1992) Immunolocalization of acidic and basic fibroblast growth factors during mouse odontogenesis. Int J Dev Biol 36:381–389
Hidaka T, Nagasawa T, Shirai K et al (2012) FGF‑2 induces proliferation of human periodontal ligament cells and maintains differentiation potentials of STRO-1(+)/CD146(+) periodontal ligament cells. Arch Oral Biol 57:830–840. https://doi.org/10.1016/j.archoralbio.2011.12.003
Xiao G, Jiang D, Gopalakrishnan R, Franceschi RT (2002) Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem 277:36181–36187. https://doi.org/10.1074/jbc.M206057200
Grimm S, Wolff E, Walter C et al (2020) Influence of clodronate and compressive force on IL-1ß-stimulated human periodontal ligament fibroblasts. Clin Oral Investig 24:343–350. https://doi.org/10.1007/s00784-019-02930-z
Pitzurra L, Jansen IDC, de Vries TJ et al (2020) Effects of L‑PRF and A‑PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res 55:287–295. https://doi.org/10.1111/jre.12714
Fukuba S, Akizuki T, Matsuura T et al (2021) Effects of combined use of recombinant human fibroblast growth factor‑2 and β‑tricalcium phosphate on ridge preservation in dehiscence bone defects after tooth extraction: a split-mouth study in dogs. J Periodontal Res 56:298–305. https://doi.org/10.1111/jre.12818
Bizenjima T, Irokawa D, Tanaka K et al (2021) Periodontal regenerative therapy with recombinant human fibroblast growth factor‑2 and deproteinized bovine bone mineral in patient with chronic periodontitis: an 18-month follow-up report. Bull Tokyo Dent Coll. https://doi.org/10.2209/tdcpublication.2020-0034
Ornitz D, Itoh N (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol 4:215–266. https://doi.org/10.1002/wdev.176
Kirschneck C, Küchler EC, Wolf M et al (2019) Effects of the highly COX-2-selective analgesic NSAID etoricoxib on human periodontal ligament fibroblasts during compressive orthodontic mechanical strain. Mediators Inflamm. https://doi.org/10.1155/2019/2514956
Niederau C, Craveiro RB, Azraq I et al (2020) Selection and validation of reference genes by RT-qPCR for murine cementoblasts in mechanical loading experiments simulating orthodontic forces in vitro. Sci Rep 10:10893. https://doi.org/10.1038/s41598-020-67449-w
Symmank J, Chorus M, Appel S et al (2020) Distinguish fatty acids impact survival, differentiation and cellular function of periodontal ligament fibroblasts. Sci Rep 10:15706. https://doi.org/10.1038/s41598-020-72736-7
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(‑Delta Delta C(T)) Method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Ishikawa S, Iwasaki K, Komaki M, Ishikawa I (2004) Role of ascorbic acid in periodontal ligament cell differentiation. J Periodontol 75:709–716. https://doi.org/10.1902/jop.2004.75.5.709
Mimori K, Komaki M, Iwasaki K, Ishikawa I (2007) Extracellular signal-regulated kinase 1/2 is involved in ascorbic acid-induced osteoblastic differentiation in periodontal ligament cells. J Periodontol 78:328–334. https://doi.org/10.1902/jop.2007.060223
Andrade I, Taddei SRA, Souza PEA (2012) Inflammation and tooth movement: the role of cytokines, chemokines, and growth factors. Semin Orthod 18:257–269. https://doi.org/10.1053/j.sodo.2012.06.004
Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. J Cell Commun Signal 3:311–322. https://doi.org/10.1007/s12079-009-0068-0
Baghdadi D, Reimann S, Keilig L et al (2019) Biomechanical analysis of initial incisor crowding alignment in the periodontally reduced mandible using the finite element method. J Orofac Orthop 80:184–193. https://doi.org/10.1007/s00056-019-00179-5
An S, Huang X, Gao Y et al (2015) FGF‑2 induces the proliferation of human periodontal ligament cells and modulates their osteoblastic phenotype by affecting Runx2 expression in the presence and absence of osteogenic inducers. Int J Mol Med 36:705–711. https://doi.org/10.3892/ijmm.2015.2271
Yun Y‑R, Won JE, Jeon E et al (2010) Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. https://doi.org/10.4061/2010/218142
Barrientos S, Stojadinovic O, Golinko MS et al (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601. https://doi.org/10.1111/j.1524-475X.2008.00410.x
Poole A, Knowland N, Cooper E et al (2014) Transitory FGF treatment results in the long-lasting suppression of the proliferative response to repeated FGF stimulation. J Cell Biochem 115:874–888. https://doi.org/10.1002/jcb.24731
Takayama S, Murakami S, Nozaki T et al (1998) Expression of receptors for basic fibroblast growth factor on human periodontal ligament cells. J Periodontal Res 33:315–322. https://doi.org/10.1111/j.1600-0765.1998.tb02205.x
Shon W‑J, Bae K‑S, Baek S‑H et al (2012) Effects of calcium phosphate endodontic sealers on the behavior of human periodontal ligament fibroblasts and MG63 osteoblast-like cells. J Biomed Mater Res B Appl Biomater 100:2141–2147. https://doi.org/10.1002/jbm.b.32779
Takahashi M, Okubo N, Chosa N et al (2012) Fibroblast growth factor-1-induced ERK1/2 signaling reciprocally regulates proliferation and smooth muscle cell differentiation of ligament-derived endothelial progenitor cell-like cells. Int J Mol Med 29:357–364. https://doi.org/10.3892/ijmm.2011.847
Tamura I, Chaqour B, Howard PS et al (2008) Effect of fibroblast growth factor‑1 on the expression of early growth response‑1 in human periodontal ligament cells. J Periodontal Res 43:305–310. https://doi.org/10.1111/j.1600-0765.2007.01030.x
LaVallee TM, Prudovsky IA, McMahon GA et al (1998) Activation of the MAP kinase pathway by FGF‑1 correlates with cell proliferation induction while activation of the Src pathway correlates with migration. J Cell Biol 141:1647–1658. https://doi.org/10.1083/jcb.141.7.1647
Ramos C, Becerril C, Montaño M, García-De-Alba C, Ramírez R, Checa M, Pardo A, Selman M (2010) FGF‑1 reverts epithelial-mesenchymal transition induced by TGF-β1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol 299(2):L222–L231. https://doi.org/10.1152/ajplung.00070.2010
Tomokiyo A, Hynes K, Ng J et al (2017) Generation of neural crest-like cells from human periodontal ligament cell-derived induced pluripotent stem cells. J Cell Physiol 232:402–416. https://doi.org/10.1002/jcp.25437
Kim SS, Kwon D‑W, Im I et al (2012) Differentiation and characteristics of undifferentiated mesenchymal stem cells originating from adult premolar periodontal ligaments. Korean J Orthod 42:307–317. https://doi.org/10.4041/kjod.2012.42.6.307
Jacobs C, Grimm S, Ziebart T et al (2013) Osteogenic differentiation of periodontal fibroblasts is dependent on the strength of mechanical strain. Arch Oral Biol 58:896–904. https://doi.org/10.1016/j.archoralbio.2013.01.009
Miller I, Min M, Yang C et al (2018) Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep 24:1105–1112
Yan Y, Zeng W, Song S et al (2013) Vitamin C induces periodontal ligament progenitor cell differentiation via activation of ERK pathway mediated by PELP1. Protein Cell 4:620–627. https://doi.org/10.1007/s13238-013-3030-0
An S, Gao Y, Ling J (2015) Characterization of human periodontal ligament cells cultured on three-dimensional biphasic calcium phosphate scaffolds in the presence and absence of L‑ascorbic acid, dexamethasone and β‑glycerophosphate in vitro. Exp Ther Med 10:1387–1393. https://doi.org/10.3892/etm.2015.2706
Boyce ST, Supp AP, Swope VB, Warden GD (2002) Vitamin C regulates keratinocyte viability, epidermal barrier, and basement membrane in vitro, and reduces wound contraction after grafting of cultured skin substitutes. J Invest Dermatol 118:565–572. https://doi.org/10.1046/j.1523-1747.2002.01717.x
Mohammed BM, Fisher BJ, Kraskauskas D et al (2016) Vitamin C promotes wound healing through novel pleiotropic mechanisms. Int Wound J 13:572–584. https://doi.org/10.1111/iwj.12484
Phillips CL, Combs SB, Pinnell SR (1994) Effects of ascorbic acid on proliferation and collagen synthesis in relation to the donor age of human dermal fibroblasts. J Invest Dermatol 103:228–232. https://doi.org/10.1111/1523-1747.ep12393187
Zeng L‑H, Wang Q‑M, Feng L‑Y et al (2019) High-dose vitamin C suppresses the invasion and metastasis of breast cancer cells via inhibiting epithelial-mesenchymal transition. Onco Targets Ther 12:7405–7413. https://doi.org/10.2147/OTT.S222702
Lam NT, Tandon I, Balachandran K (2019) The role of fibroblast growth factor 1 and 2 on the pathological behavior of valve interstitial cells in a three-dimensional mechanically-conditioned model. J Biol Eng 13:45. https://doi.org/10.1186/s13036-019-0168-1
Rossini M, Cheunsuchon B, Donnert E et al (2005) Immunolocalization of fibroblast growth factor‑1 (FGF-1), its receptor (FGFR-1), and fibroblast-specific protein‑1 (FSP-1) in inflammatory renal disease. Kidney Int 68:2621–2628. https://doi.org/10.1111/j.1523-1755.2005.00734.x
Arias-Gallo J, Chamorro-Pons M, Avendaño C, Giménez-Gallego G (2013) Influence of acidic fibroblast growth factor on bone regeneration in experimental cranial defects using spongostan and Bio-Oss as protein carriers. J Craniofac Surg 24:1507–1514. https://doi.org/10.1097/SCS.0b013e31828f2469
Shiga M, Kapila YL, Zhang Q et al (2003) Ascorbic acid induces collagenase‑1 in human periodontal ligament cells but not in MC3T3-E1 osteoblast-like cells: potential association between collagenase expression and changes in alkaline phosphatase phenotype. J Bone Miner Res 18:67–77. https://doi.org/10.1359/jbmr.2003.18.1.67
David JM, Dominguez C, Hamilton DH, Palena C (2016) The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines 4:22. https://doi.org/10.3390/vaccines4030022
Srivastava SK, Tetsuka T, Daphna-Iken D, Morrison AR (1994) IL‑1 beta stabilizes COX II mRNA in renal mesangial cells: role of 3’-untranslated region. Am J Physiol 267:F504–F508. https://doi.org/10.1152/ajprenal.1994.267.3.F504
Jang B‑C, Muñoz-Najar U, Paik J‑H et al (2003) Leptomycin B, an inhibitor of the nuclear export receptor CRM1, inhibits COX‑2 expression. J Biol Chem 278:2773–2776. https://doi.org/10.1074/jbc.C200620200
Ristimäki A, Garfinkel S, Wessendorf J et al (1994) Induction of cyclooxygenase‑2 by interleukin‑1 alpha. Evidence for post-transcriptional regulation. J Biol Chem 269:11769–11775
Kang Y‑G, Nam J‑H, Kim K‑H, Lee K‑S (2010) FAK pathway regulates PGE2 production in compressed periodontal ligament cells. J Dent Res 89:1444–1449. https://doi.org/10.1177/0022034510378521
Acknowledgements
The authors thank the Medical Faculty of Jena, Germany, for scientific support. In addition, the authors thank Katrin von Brandenstein for her technical support. Parts of this study were awarded with the Best Presented Poster Award (Parallel Symposium) at the 92nd Scientific Annual Meeting of the German Orthodontic Society in Nuremberg, Germany 2019.
Funding
This work was supported by a grant from the Interdisciplinary Center for Clinical Research within the faculty of Medicine at the RWTH Aachen University (OC1‑1, OC1‑2, OC1-3) and the German Orthodontic Society (DGKFO).
Author information
Authors and Affiliations
Contributions
I. Knaup designed the study, performed experiments, data analysis and wrote the manuscript, J. Symmank designed the study, performed experiments, data analysis, figure illustration and wrote the manuscript, A. Bastian performed experiments, data analysis and critically revised the manuscript, S. Neuss, T. Pufe, C. Jacobs critically revised the manuscript, and M. Wolf designed the study and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
I. Knaup, J. Symmank, A. Bastian, S. Neuss, T. Pufe, C. Jacobs and M. Wolf declare that they have no financial or nonfinancial competing interests.
Ethical standards
This article does not report on any studies with human participants or animals that were performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Isabel Knaup and Judit Symmank contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Knaup, I., Symmank, J., Bastian, A. et al. Impact of FGF1 on human periodontal ligament fibroblast growth, osteogenic differentiation and inflammatory reaction in vitro. J Orofac Orthop 83 (Suppl 1), 42–55 (2022). https://doi.org/10.1007/s00056-021-00363-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00056-021-00363-6