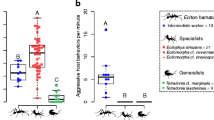
Abstract
Mutualistic, commensalistic or parasitic interactions are unevenly distributed across the animals and plants: in certain taxa, such interspecific associations evolved more often than in others. Within the ants, associations between species of the genera Camponotus and Crematogaster evolved repeatedly and include trail-sharing associations, where two species share foraging trails, and parabioses, where two species share a nest without aggression. Camponotus and Crematogaster may possess life-history traits that favour the evolution of associations. To identify which traits are affected by the association, we investigated a neotropical parabiosis of Ca. femoratus and Cr. levior and compared it to a paleotropical parabiosis and a trail-sharing association. The two neotropical species showed altered cuticular hydrocarbon profiles compared to non-parabiotic species accompanied by low levels of interspecific aggression. Both species occurred in two chemically distinct types. Camponotus followed artificial trails of Crematogaster pheromones, but not vice versa. The above traits were also found in the paleotropical parabiosis, and the trail-following results match those of the trail-sharing association. In contrast to paleotropical parabioses, however, Camponotus was dominant, had a high foraging activity and often fought against Crematogaster over food resources. We suggest three potential preadaptations for parabiosis. First, Crematogaster uses molecules as trail pheromones, which can be perceived by Camponotus, too. Second, nests of Camponotus are an important benefit to Crematogaster and may create a selection pressure for the latter to tolerate Camponotus. Third, there are parallel, but unusual, shifts in cuticular hydrocarbon profiles between neotropics and paleotropics, and between Camponotus and Crematogaster.



Similar content being viewed by others
References
Adams ES (1990) Interaction between the ants Zacryptocerus maculatus and Azteca trigona: interspecific parasitization of information. Biotropica 22:200–206
Akino T (2002) Chemical camouflage by myrmecophilous beetles Zyras comes (Coleoptera: staphylinidae) and Diaritiger fossulatus (Coleoptera : Pselaphidae) to be integrated into the nest of Lasius fuliginosus (Hymenoptera : Formicidae). Chemoecology 12:83–89
Attygalle AB, Morgan ED (1985) Ant trail pheromones. Adv Insect Physiol 18:1–30
Boulay R, Cerdá X, Simon T, Roldan M, Hefetz A (2007) Intraspecific competition in the ant Camponotus cruentatus: should we expect the ‘dear enemy’ effect? Anim Behav 74:985–993
Bronstein JL (2001) The exploitation of mutualisms. Ecol Lett 4:277–287
Buczkowski G, Silverman J (2005) Context-dependent nestmate discrimination and the effect of action thresholds on exogenous cue recognition in the Argentine ant. Anim Behav 69:741–749
Buschinger A (2009) Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol News 12:219–235
Carlin NF, Hölldobler B (1983) Nestmate and kin recognition in interspecific mixed colonies of ants. Science 222:1027–1029
Carroll CR, Janzen DH (1973) Ecology of foraging by ants. Annu Rev Ecol Syst 4:231–257
Davidson DW (1988) Ecological studies of neotropical ant gardens. Ecology 69:1138–1152
Dejean A (1996) Trail sharing in African arboreal ants. Sociobiology 27:1–9
D’Ettorre P, Heinze J (2001) Sociobiology of slave-making ants. Acta Ethol 3:67–82
D’Ettorre P, Errard C, Ibarra F, Francke W, Hefetz A (2000) Sneak in or repel your enemy: Dufour’s gland repellent as a strategy for successful usurpation in the slave-maker Polyergus rufescens. Chemoecology 10:135–142
Emery V, Tsutsui ND (2013) Recognition in a social symbiosis: chemical phenotypes and nestmate recognition behaviors of neotropical parabiotic ants. PLoS One 8:e56492
Errard C, Regla JI, Hefetz A (2003) Interspecific recognition in Chilean parabiotic ant species. Insectes Soc 50:268–273
Espadaler X, Martí S (1994) La feromona de pista de Crematogaster Lund (Hymenoptera, Formicidae): Vàlida per tot el gènere? Sessió d’Entomologia ICHN-SCL 8:81–86
Fiedler K (1998) Lycaenid-ant interactions of the Maculinea type: tracing their historical roots in a comparative framework. J Insect Conserv 2:3–14
Forel A (1898) La parabiose chez les fourmis. Bull Soc Vaudoise des Sci Nat 34:380–384
Geiselhardt SF, Peschke J, Nagel P (2007) A review of myrmecophily in ant nest beetles (Coleoptera: Carabidae: Paussinae): linking early observations with recent findings. Naturwissenschaften 94:871–894
Gobin B, Peeters C, Billen J, Morgan ED (1998) Interspecific trail following and commensalism between the ponerine ant Gnamptogenys menadensis and the formicine ant Polyrhachis rufipes. J Insect Behav 11:361–369
Hölldobler B, Wilson EO (1990) The ants. Springer, Berlin Heidelberg
Hölldobler B, Möglich M, Maschwitz U (1981) Myrmecophilic relationship of Pella (Coleoptera: Staphylinidae) to Lasius fuliginosus (Hymenoptera: Formicidae). Psyche 88:347–374
Katzav-Gozansky T, Boulay R, Ionescu-Hirsh A, Hefetz A (2008) Nest volatiles as modulators of nestmate recognition in the ant Camponotus fellah. J Insect Physiol 54:378–385
Kaufmann E, Maschwitz U (2006) Ant-gardens of tropical Asian rainforests. Naturwissenschaften 93:216–227
Kaufmann E, Malsch AKF, Erle M, Maschwitz U (2003) Compound nesting of Strumigenys sp. (Myrmicinae) and Diacamma sp. (Ponerinae), and other nesting symbioses of myrmicine and ponerine ants in Southeast Asia. Insectes Soc 50:88–97
Kistner DH (1979) Social and evolutionary significance of social insect symbionts. In: Hermann HR (ed) Social insects. Academic Press, New York, San Francisco, London, pp 339–413
Knaden M, Wehner R (2003) Nest defense and conspecific enemy recognition in the desert ant Cataglyphis fortis. J Insect Behav 16:717–730
Kroiss J, Svatoš A, Kaltenpoth M (2011) Rapid identification of insect CHCs using gas chromatography—ion-trap mass spectrometry. J Chem Ecol 27:420–427
Lambardi D, Dani FR, Turillazzi S, Boomsma JJ (2007) Chemical mimicry in an incipient leaf-cutting ant social parasite. Behav Ecol Sociobiol 61:843–851
Lenoir A, D’Ettorre P, Errard C, Hefetz A (2001) Chemical ecology and social parasitism in ants. Annu Rev Entomol 46:573–599
Longino JT (2003) The Crematogaster (Hymenoptera, Formicidae, Myrmicinae) of Costa Rica. Zootaxa 151:1–150
Lutzoni F, Pagel M, Reeb V (2001) Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411:937–940
Menzel F, Blüthgen N (2010) Parabiotic associations between tropical ants: equal partnership or parasitic exploitation? J Anim Ecol 79:71–81
Menzel F, Schmitt T (2011) Tolerance requires the right smell: first evidence for interspecific selection on chemical recognition cues. Evolution 66–3:896–904
Menzel F, Blüthgen N, Schmitt T (2008a) Tropical parabiotic ants: highly unusual cuticular substances and low interspecific discrimination. Front Zool 5:16
Menzel F, Linsenmair KE, Blüthgen N (2008b) Selective interspecific tolerance in tropical Crematogaster–Camponotus associations. Anim Behav 75:837–846
Menzel F, Schmitt T, Blüthgen N (2009) Intraspecific nestmate recognition in two parabiotic ant species: acquired recognition cues and low inter-colony discrimination. Insectes Soc 56:251–260
Menzel F, Pokorny T, Blüthgen N, Schmitt T (2010a) Trail-sharing among tropical ants: interspecific use of trail pheromones? Ecol Entomol 35:495–503
Menzel F, Woywod M, Blüthgen N, Schmitt T (2010b) Behavioural and chemical mechanisms behind a Mediterranean ant–ant association. Ecol Entomol 35:711–720
Menzel F, Staab M, Chung AYC, Gebauer G, Blüthgen N (2012) Trophic ecology of parabiotic ants: do the partners have similar food niches? Austral Ecol 37:537–546
Menzel F, Blüthgen N, Tolasch T, Conrad J, Beifuß U, Beuerle T, Schmitt T (2013) Crematoenones—a novel substance class exhibited by ants functions as appeasement signal. Front Zool 10:32
Moneti G, Pieraccini G, Dani F, Turillazzi S, Favretto D, Traldi P (1997) Ion-molecule reactions of ionic species from acetonitrile with unsaturated hydrocarbons for the identification of the double-bond position using an ion trap. J Mass Spectrom 32:1371–1373
Morgan ED (2009) Trail pheromones of ants. Physiol Entomol 34:1–17
Morgan ED, Brand JM, Mori K, Keegans SJ (2004) The trail pheromone of the ant Crematogaster castanea. Chemoecology 14:119–120
Oettler J, Schmitt T, Herzner G, Heinze J (2008) Chemical profiles of mated and virgin queens, egg-laying intermorphs and workers of the ant Crematogaster smithi. J Insect Physiol 54:672–679
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2011) Community ecology package: package ‘vegan’ 2.0-0. www.r-project.org
Oldham NJ, Svatoš A (1999) Determination of the double bond position in functionalized monoenes by chemical ionization ion-trap mass spectrometry using acetonitrile as a reagent gas. Rapid Commun Mass Spectrom 13:331–336
Orivel J, Dejean A (1999) Selection of epiphyte seeds by ant-garden ants. Ecoscience 6:51–55
Orivel J, Leroy C (2010) The diversity and ecology of ant gardens (Hymenoptera: formicidae; Spermatophyta: Angiospermae). Myrmecol News 14:73–85
Orivel J, Errard C, Dejean A (1997) Ant gardens: interspecific recognition in parabiotic ant species. Behav Ecol Sociobiol 40:87–93
Parr CL, Gibb H (2010) Competition and the role of dominant ants. In: Lach L, Parr CL, Abbott KL (eds) Ant ecology. Oxford University Press, Oxford, pp 77–96
Santini G, Tucci L, Ottonetti L, Frizzi F (2007) Competition trade-offs in the organisation of a Mediterranean ant assemblage. Ecol Entomol 32:319–326
Seifert B (2007) Die Ameisen Mittel- und Nordeuropas. Lutra Verlags- und Vertriebsgesellschaft, Görlitz/Tauer
Swain RB (1980) Trophic competition among parabiotic ants. Insectes Soc 27:377–390
R Development Core Team (2011) R: a language and environment for statistical computing. http://www.R-project.org. R Foundation for Statistical Computing, Vienna
Vantaux A, Dejean A, Dor A, Orivel J (2007) Parasitism versus mutualism in the ant-garden parabiosis between Camponotus femoratus and Crematogaster levior. Insectes Soc 54:95–99
Weißflog A (2001) Freinestbau von Ameisen (Hymenoptera: Formicidae) in der Kronenregion feuchttropischer Wälder Südostasiens. Frankfurt am Main: PhD Thesis, J. W. Goethe-Universität
Wheeler WM (1921) A new case of parabiosis and the “ant gardens” of British Guiana. Ecology 2:89–103
Wilson EO (1965) Trail sharing in ants. Psyche 72:2–7
Wilson EO (1987) The arboreal ant fauna of Peruvian amazon forests: a first assessment. Biotropica 19:245–251
Witte V, Janssen R, Eppenstein A, Maschwitz U (2002) Allopeas myrmekophilos (Gastropoda, Pulmonata), the first myrmecophilous mollusc living in colonies of the ponerine army ant Leptogenys distinguenda (Formicidae, Ponerinae). Insectes Soc 49:301–305
Witte V, Lehmann L, Lustig A, Maschwitz U (2009) Polyrhachis lama, a parasitic ant with an exceptional mode of social integration. Insectes Soc 56:301–307
Yéo J, Molet M, Peeters C (2006) When David and Goliath share a home: compound nesting of Pyramica and Platythyrea ants. Insectes Soc 53:435–438
Acknowledgments
We are grateful to the Laboratoire Environnement de Petit Saut for furnishing logistical support and the Les Nouragues Research Station for research permission. This work has benefited from an “Investissement d’Avenir” grant managed by the Agence Nationale de la Recherche (CEBA, ref. ANR-10-LABX-0025).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental figures: Fig. S1 Substance class by chain length for each of the samples analysed. Each barplot represents one colony. The bars give the relative abundance of CHC, separated by chain length and with different colours for each substance class. The bars of each barplot add up to 100 %. Note that the graphs do not show differences between methyl group positions or (for alkenes) retention times among substances of the same chain length.
Fig. S2 NMDS ordination of CHC of Crematogaster levior, Cr. carinata and Camponotus femoratus.
Supplemental Tables Table S1. Cuticular hydrocarbons of Camponotus femoratus, Crematogaster levior and Crematogaster carinata. Each column represents one colony, with collection site and colony code given above. The numbers give the relative abundance of each substance.
Table S2. PERMANOVA results from aggression assays for the four species combinations. The aggression index was determined for the aggression of residents towards an intruder; species combination is given as ‘resident-intruder’.
Table S3. Results for foraging ecology, data from Vantaux et al. (2007).
Rights and permissions
About this article
Cite this article
Menzel, F., Orivel, J., Kaltenpoth, M. et al. What makes you a potential partner? Insights from convergently evolved ant–ant symbioses. Chemoecology 24, 105–119 (2014). https://doi.org/10.1007/s00049-014-0149-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0149-2